Copolymers of ethylene and selected acrylate esters
a technology of acrylate esters and copolymers, which is applied in the direction of catalytic reactions, group 3/13 element organic compounds, group 5/15 element organic compounds, etc., can solve the problems of long delay between, high cost of analysis, and adjustment of manufacturing systems to minimize homopolymer formation or adjust the level of acrylate incorporation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
1-5
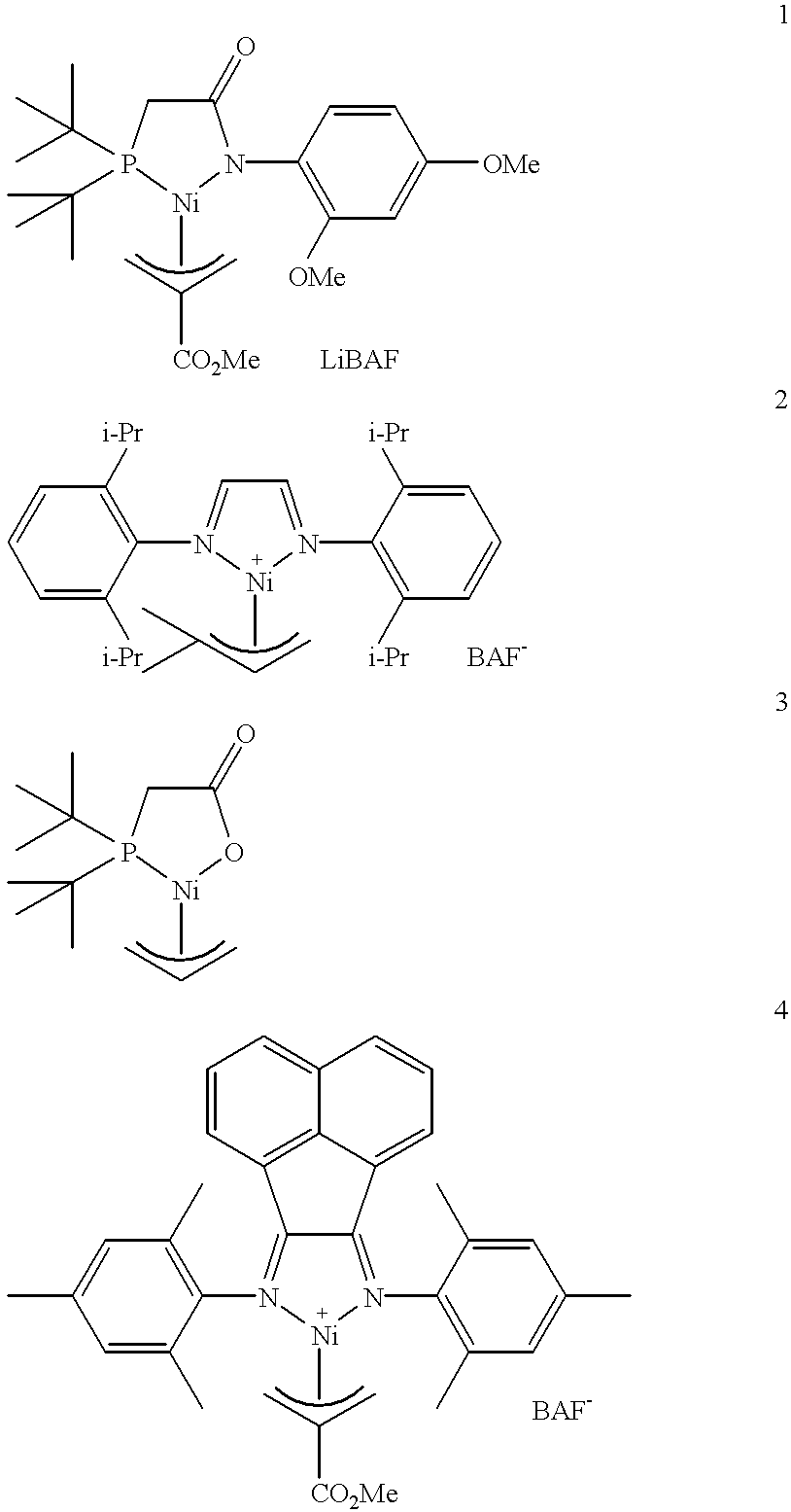
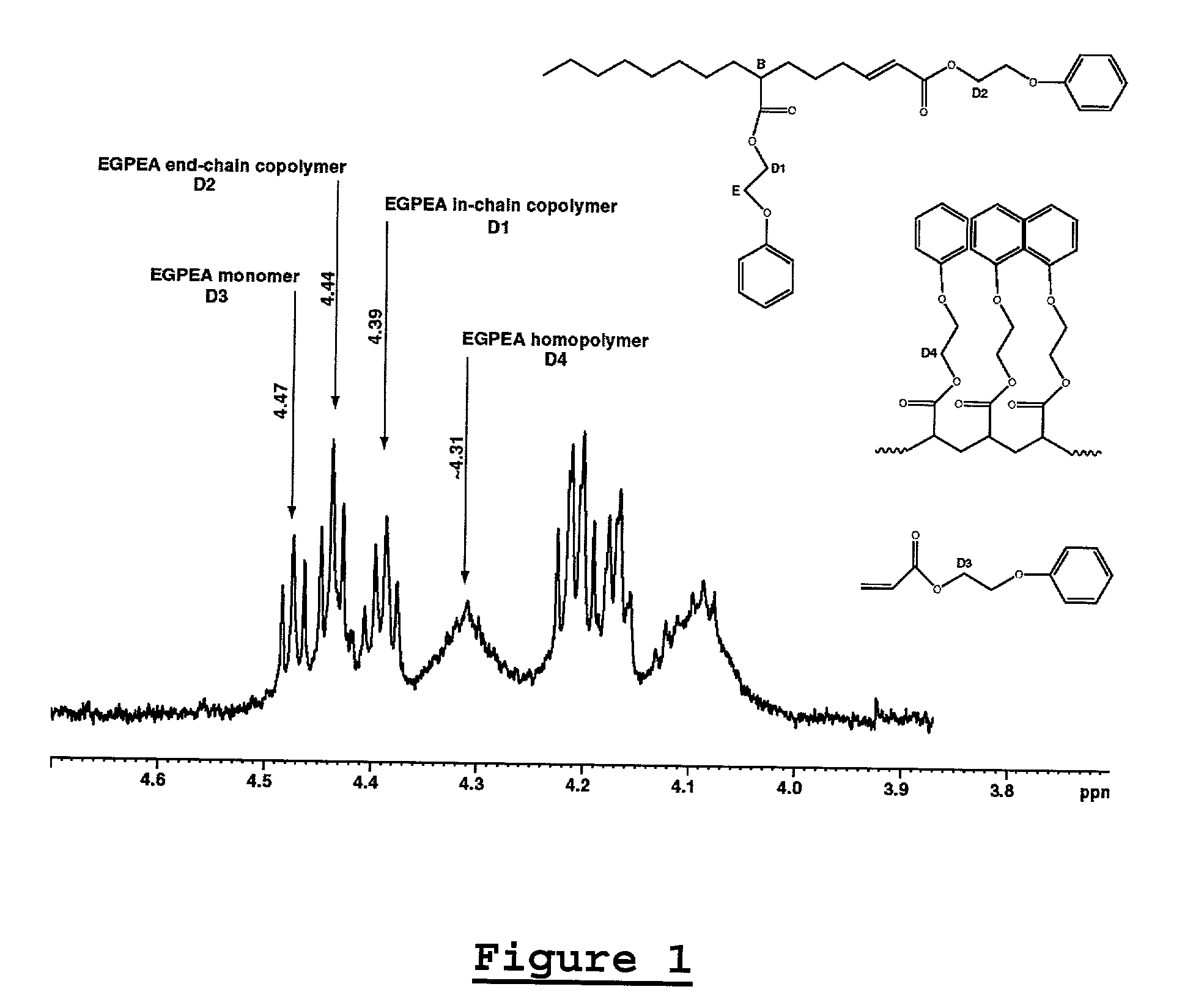
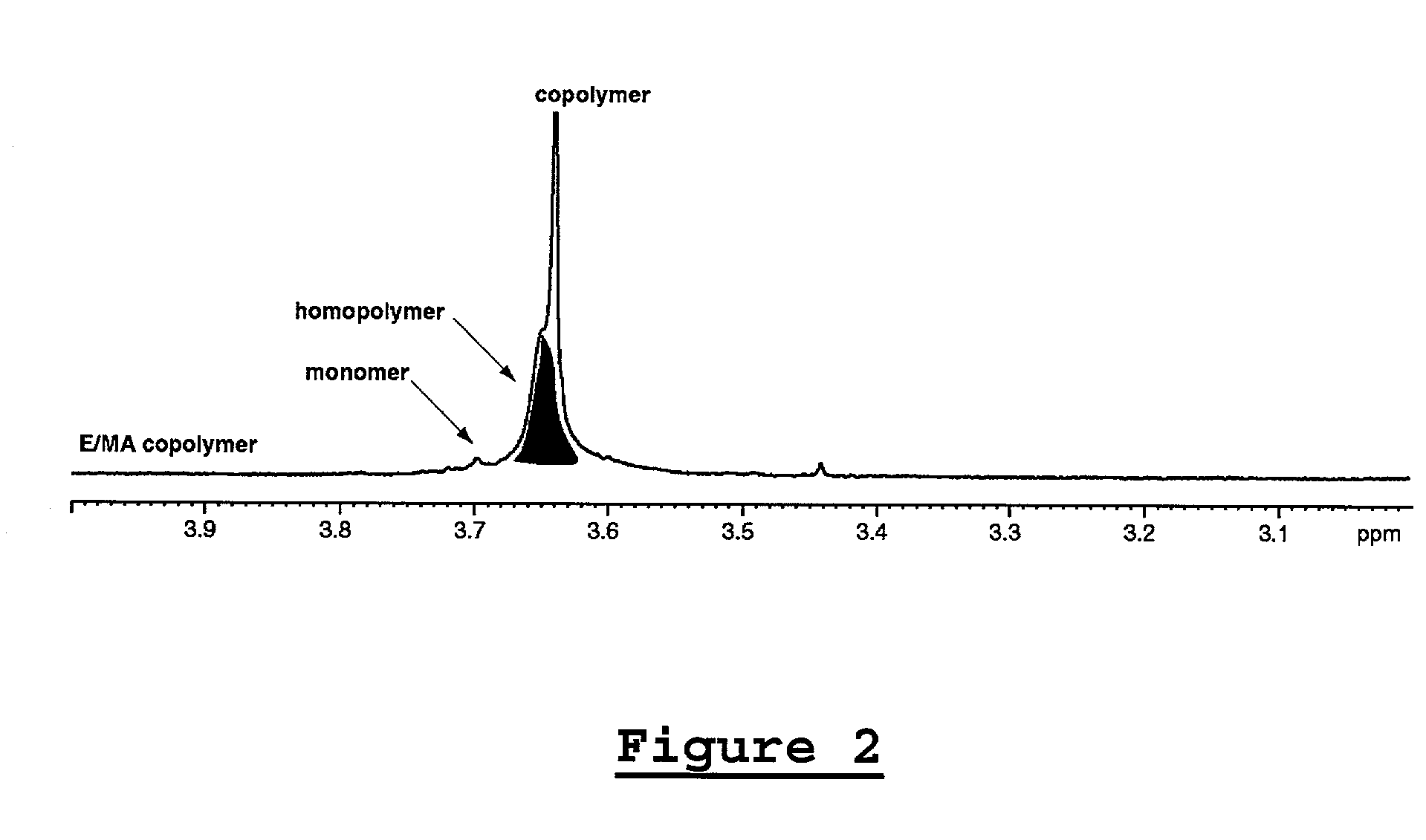
[0129] Examples 1-5 are listed in Tables 1 and 2 below. Figures for compounds 1 through 4 are shown above. The polymerizations were carried out according to General Procedure A. Varying amounts of acrylate homopolymer are present in the isolated polymers. In Table 1, the yield of the polymer is reported in grams and includes the yield of the dominant ethylene / acrylate copolymer as well as the yield of any acrylate homopolymer that was formed. Molecular weights were determined by GPC, unless indicated otherwise. Mole percent acrylate incorporation and total Me were determined by .sup.1H NMR spectroscopy, unless indicated otherwise. Mole percent acrylate incorporation is typically predominantly IC, unless indicated otherwise.
1TABLE 1 E / Acrylate Copolymerizations (6.9 MPa E, 120.degree. C., 18 h) Acrylate mL B(C.sub.6F.sub.5).sub.3 Acrylate Homo- Cmpd (Solvent (Borate Yield.sup.a Incorp. Total polymer.sup.b g Ex (mmol) mL) equiv) g mol % M.W. Me (.sup.13C NMR) 1 1 EGPEA 2 20 equiv 8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com