"prionins", highly specific markers for noninvasive pre-symptomatic detection of tse diseases, and targets for therapeutic reagents to prevent and control tse diseases in animals and humans
a tse disease and highly specific technology, applied in the field of prionins, can solve the problems of unsatisfactory explanation and unsubstantial progress in these directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0026] Detection of BSAS, SCRAPAS and CJAS in clinical and other samples.
[0027] Prionin proteins were detected using the ELISAs described above. Since some populations ft the polyclonal anti-TSE antibodies cross react with domains of the major epitopes of each TSE species, with varying degrees of sensitivity, anti-SCRAPAS-HRP is used as the second antibody for detecting both BSAS and SCHAPAS, whereas either anti-SCRAPAS HRP or anti-CJAS-HRP is used as the second antibody for detecting CJAS.
example 3
[0028] Using another approach, which is not suitable for use as a routine test method (with BSAS as the example) : BSAS protein was isolated from 100 .mu.l of serum, from an infected cow on a anti-BSAS antibody immuno-affinity column. The isolated protein was subjected to SDS gel electrophoresis as described by Schgger, H. and von Jabow, G. I. Anal. Biochem. 166:368-379 (1987) or by non-SDS PAGE. Following electrophoresis the proteins were subjected to Western blotting or spotted onto nylon membrane and treated with the affinity purified antibody. Interaction of the antibody with the protein bound to the membrane was visualized with a chemiluminescent kit purchased from Bio-Rad Inc. according to the manufacturer's instructions (also see Blake M. S. et al.Anal biochem. 136:175-179 (1984)). MOPAS and HAMPAS were discovered using the same procedure; however, the expression of these two proteins in animals with experimental TSE disease have not been investigated so far.
example 4
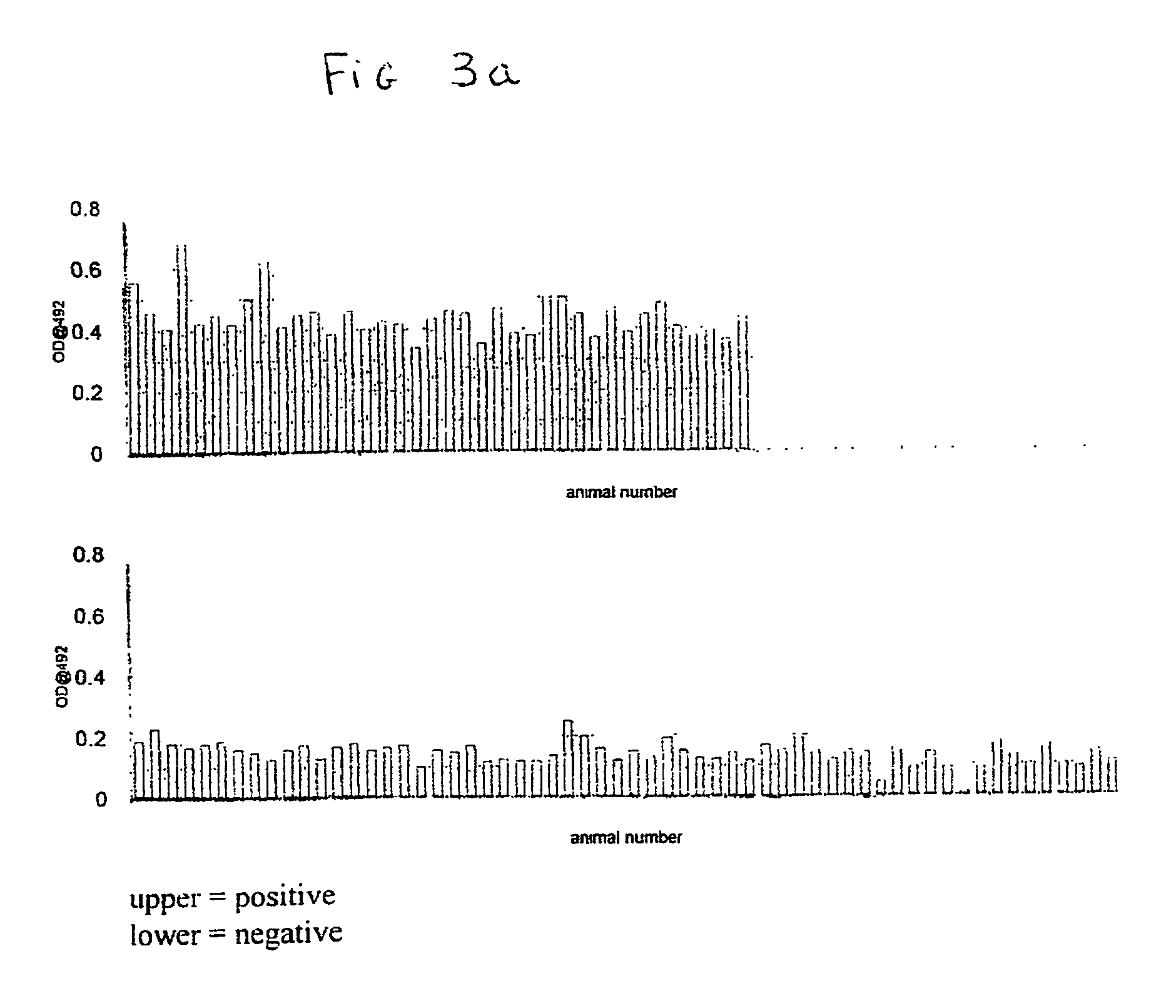
[0029] We have demonstrated, in the case of BSAS and SCHAPAS, a specific association (100%) of the proteins with animals confirmed with the disease or exposed in any manner to the disease; whereas the proteins were not associated with animals which have never been exposed to the disease. BSAS and SCHAPAS was detected in serum of all animals clinically positive for the diseases and in the majority of animal that had no demonstrated clinical symptoms of the disease which came from herds that had even a single case of the disease. CJAS was detected in serum of two CJD victims in one of which was positive for both CJD and Alzheimer's disease.
[0030] The success of the present invention derived from our ability to find alternative genes encoding potentially pathogenic proteins BSAS, SCRAPAS and CJAS within the PrP genes of cattle, sheep and humans. The discovery of equivalent genes in mice and hamsters contributed to formulating our model for TSE.
Details of the Invention
[0031] In detail t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com