Agents and compositions and methods utilizing same useful in diagnosing and/or treating or preventing plaque forming
a technology of agent composition and plaque forming, which is applied in the direction of antibody mimetics/scaffolds, peptide/protein ingredients, fusion polypeptides, etc., can solve the problems of teaching not providing means with which to enable, and the physiochemical properties of abnormalities are abnormal, and the effect of increasing the number o
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Generation of an IgM Hybridoma 508
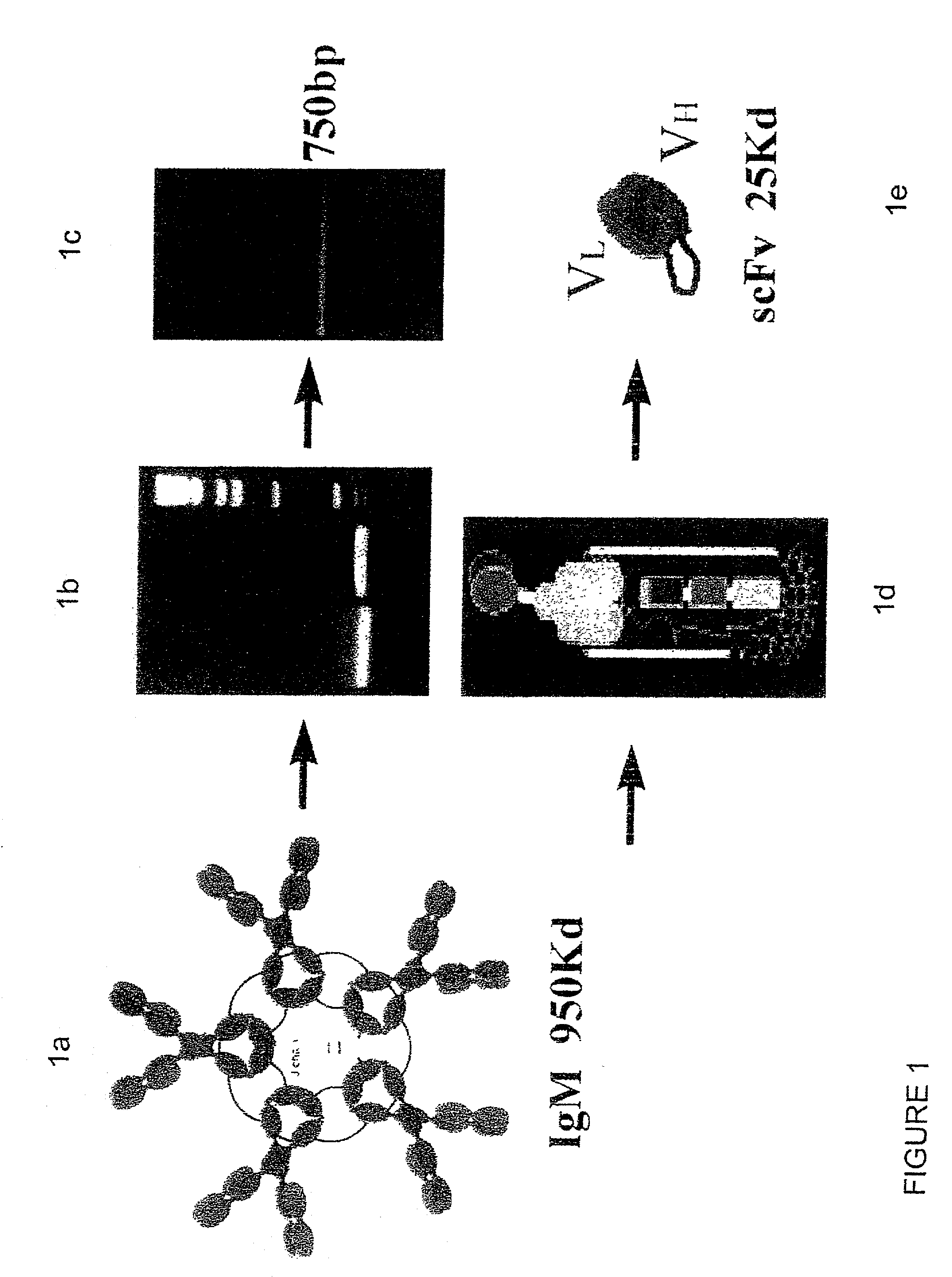
[0240] Immunization of a mouse with a 16 amino acid peptide of beta-amyloid (acids 1-16 of SEQ ID NO: 3) conjugated to KLH (SEQ ID NO: 9) was carried out as described hereinabove. Repetitious immunization eventually produced a low but measurable antibody titer against beta-amyloid. Subsequent splenectomy of the immunized mouse facilitated preparation of IgM hybridoma 508 expressing scFvAb with specificity to beta-amyloid. RNA was subsequently extracted from this hybridoma. The IgM 508 hybridoma showed specific activity to A.beta. in preventing its toxic affects on PC12 cells (Anavi, S. 1998, M.Sc. thesis from the department of Molecular Microbiology and Biotechnology of the Tel-Aviv University, I).
example 2
Cloning of the Variable Domains of the 508 IgM Hybridoma as a scFv
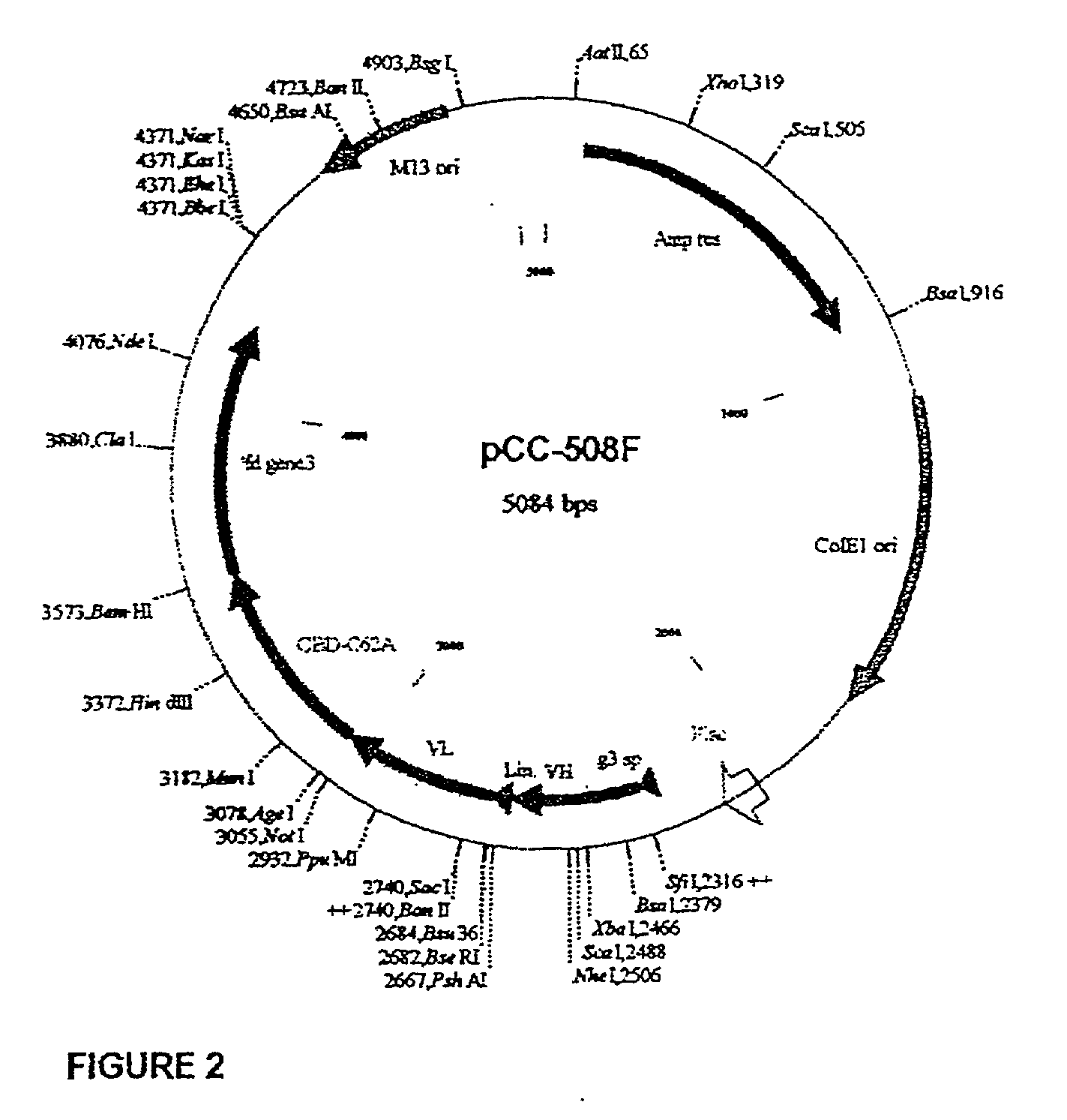
[0241] MAb 508 showed specific recognition of .beta.-amyloid and prevented its toxic affects on PC12 cells (Anavi S., 1998, ibid). For cloning the 508 antibody as a scFv in a phage display vector, RNA was extracted from 10.sup.8 508 hybridoma cells and was used as a source for antibody variable region coding sequences. RT-PCR was used to amplify the variable domains that were cloned into the phage display vector pCC-Gal6(Fv), as described in Materials and Methods. When hybridoma derived antibodies are cloned as scFvs, some of the clones may contain aberrant sequences that are not functional. Therefore, to identify phagemid clones carrying functional .beta.-amyloid binders from the generated clones, 10 individual clones were picked at random and soluble scFv-CBD fusion protein was produced thereby. FIG. 2 shows a physical map of plasmid pCC-508 which was used to express the 508-scFv. The CBD domain serves as an immunol...
example 3
Site Directed Mutagenesis of 508-(Fv) Antibody
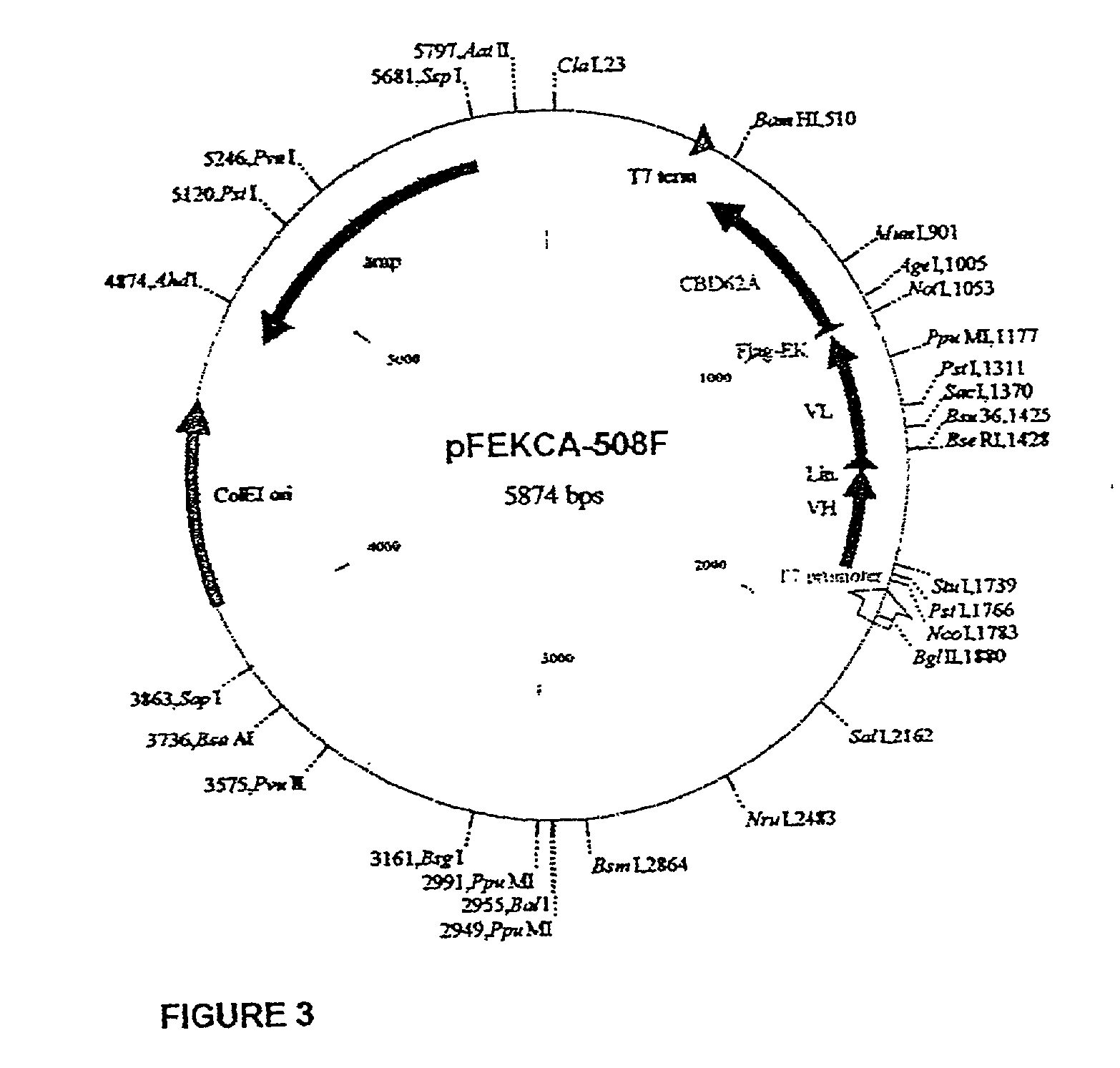
[0242] The DNA sequencing analysis of pCC508-(Fv) revealed the unusual appearance of a cysteine residue at the position 96 of V.sub.L CDR3 (Kabat, E. A. et al., Sequences of proteins of immunological interest, 5th Ed., 1991). The deduced amino acid sequence of V.sub.L CDR3 is: H.sup.89QRSSYPCT.sup.97 (SEQ ID NO: 15). The presence of an unpaired cysteine residue in a scFv may reduce its folding yield and also decrease its stability in solution and its storage half life. Therefore, 508(Fv) was subcloned into an expression vector and produced in E. coli as described in Materials and Methods. FIG. 6 summarizes the production process of 508(Fv)-CBD by the cellulose-assisted refolding method (Berdichevsky Y et al, Protein Expr Purif., 17(2):249-59, 1999). Although 508(Fv)-CBD could be purified to near homogeneity (FIG. 6 lane 7) by this method, it refolded relatively poorly and was unstable upon storage at 4.degree. C. (FIG. 7). It was assumed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com