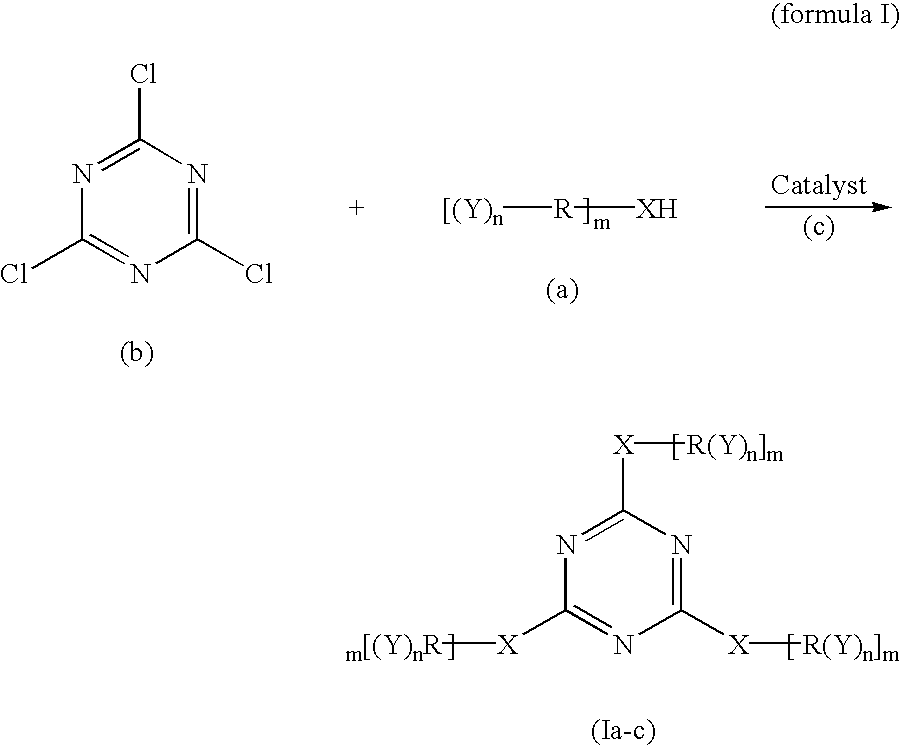
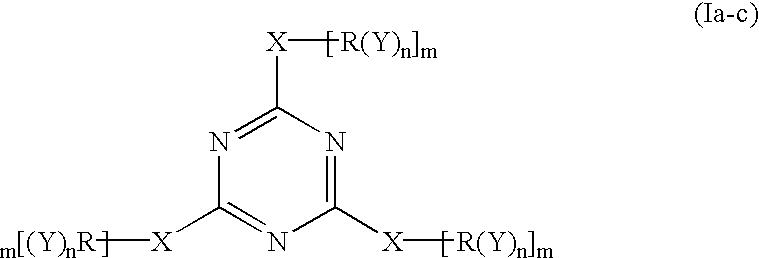
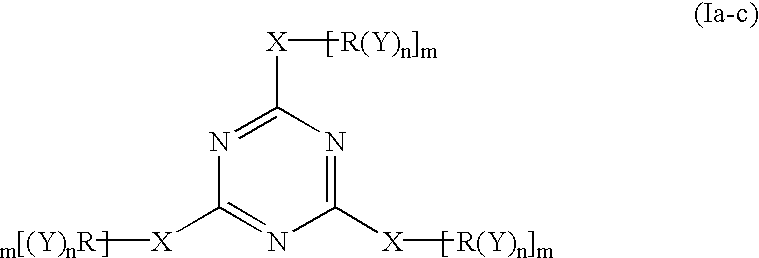
Polyether polyols with increased functionality
a polyether polyol and functional technology, applied in the field of polyether polyols with increased functionality, can solve the problems of high explosiveness, process seriousness, and inapplicability, and achieve the effects of increasing the reactivity of isocyanates, increasing the functionality, and speeding up the reaction ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0052] The following Table 1 illustrates the amount of material used in Example 1 which were reacted according to the invention to form the epoxide-reactive compound.
1 TABLE 1 Example 1 Amount Organic Compound A 300 g Cyanuric Chloride 21.5 g Solvent A: 50 mL
[0053] Organic Compound A: an organic compound started from glycerin, having a functionality of three (3), a molecular weight about of 480 and an OH number of about 351; and being prepared by reacting 1 mole of glycerin with 5 to 8 moles of PO (propylene oxide) and 7% by weight of KOH, and dewatering to give 5% by wt. potassium
[0054] Solvent A: N-methylpyrrolidinone
[0055] In Example 1 above, the cyanuric chloride was dissolved in Solvent A, thereby forming a solution. This solution was slowly added to Organic Compound A in the reaction vessel. The reaction mixture was heated to 100.degree. C. and maintained at this temperature for about 2 hours. The resulting material was filtered, and Solvent A was removed by distillation. This...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosities | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com