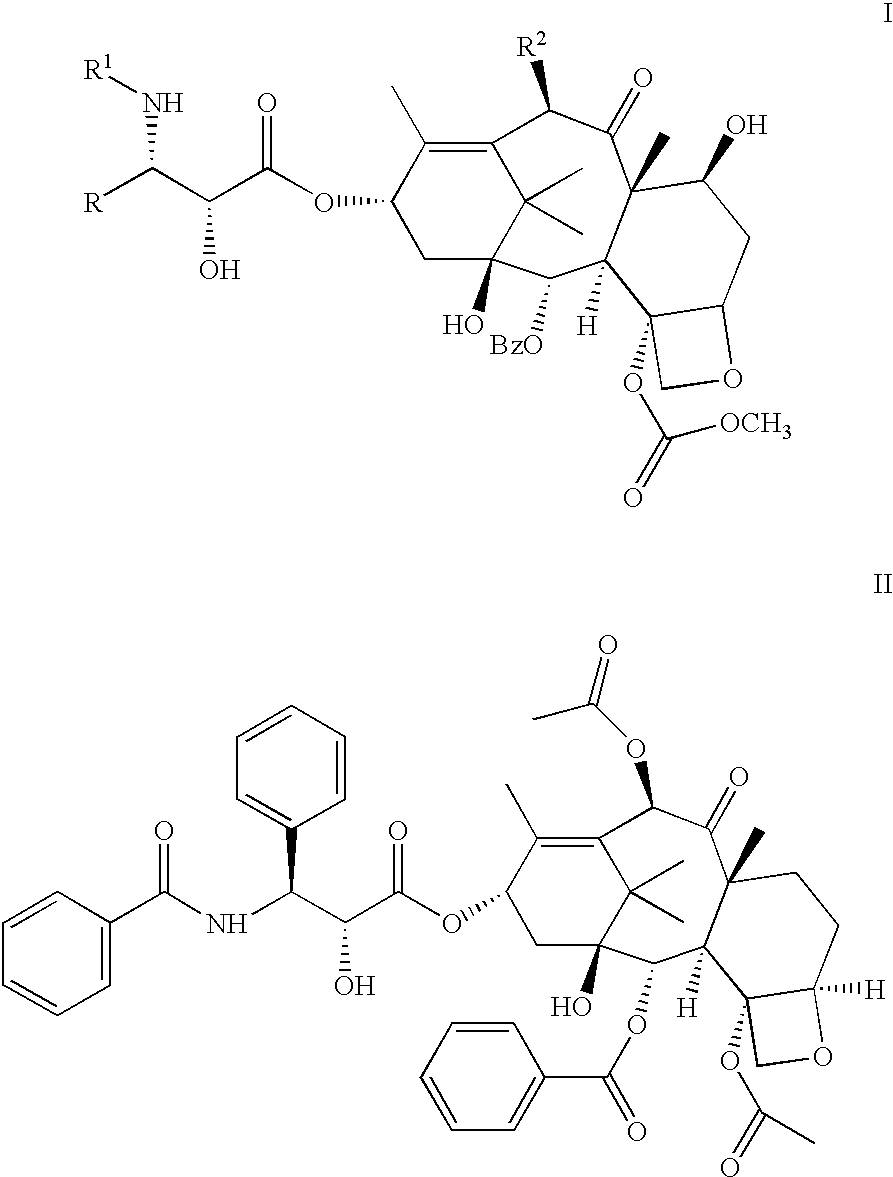
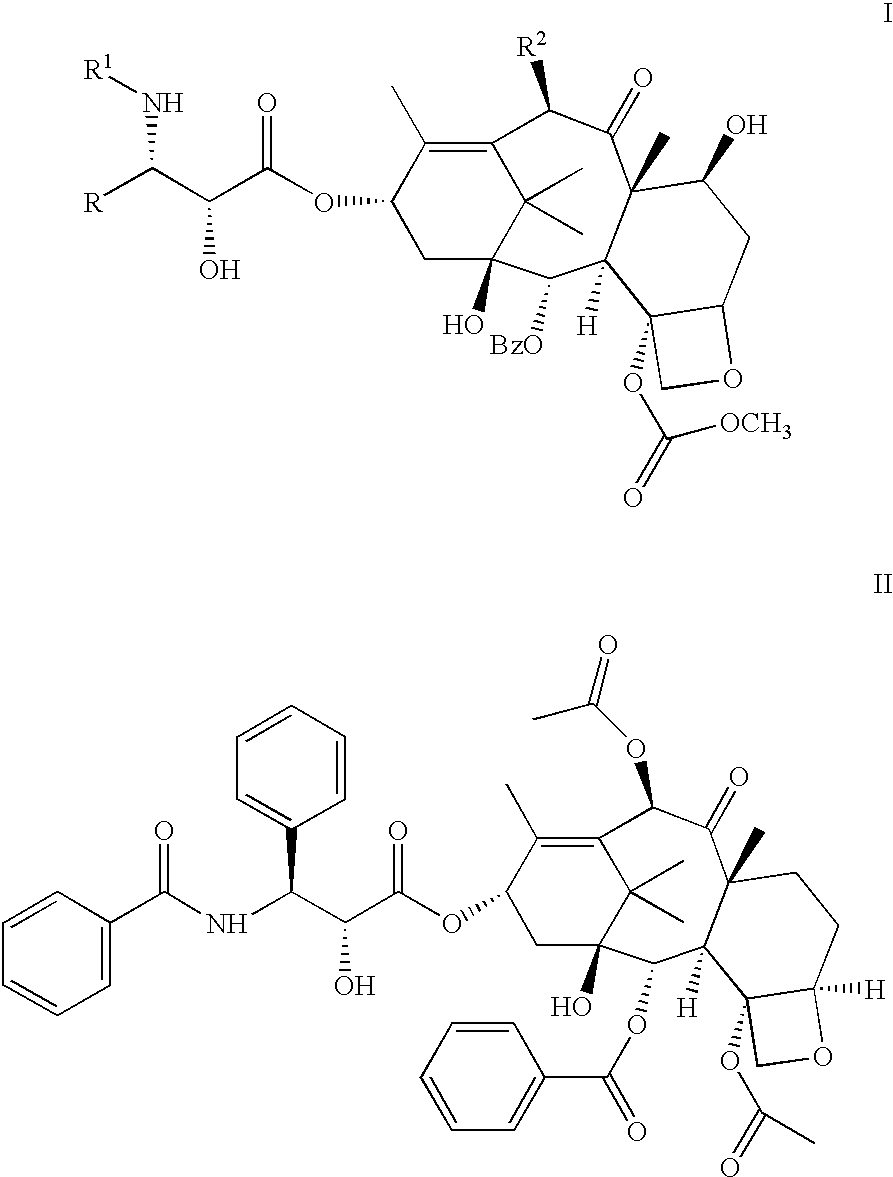
Pharmaceutical compositions of orally active taxane derivatives having enhanced bioavailability
a technology of bioavailability and composition, which is applied in the direction of drug compositions, biocide, capsule delivery, etc., can solve the problems of high interpatient variability, unsatisfactory taste, and non-linear response in absorption versus dos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0036] Compound Ia was added to a batching vessel containing polyethylene glycol 400, Tween.RTM.80, and pre-melted polyethylene glycol 1450 and mixed at about 65.degree. C. to dissolve the drug and give a solution at 4% by weight. The solution was filled into size #0 gray, opaque hard gelatin capsules at 625 mg to provide a dosage form at a strength of 25 mg of the taxane derivative per capsule. Caps were placed on the filled capsule bodies after they were stored at room temperature for about 30-60 minutes to solidify the filled contents. The recommended storage condition for the capsules is 12 months at controlled room temperature of 15-25.degree. C. (59-77.degree. F.). The dosage form exhibits high potency recovery, rapid dissolution, and maintains excellent chemical, physical and dissolution stability during long-term storage, including no evidence of drug crystallization in the semi-solid matrix. Dissolution studies in water (in the absence of added surfactant) indicate t...
example 3
[0037] Compound Ia was added to a batching vessel containing polyethylene glycol 400, pre-melted polyethylene glycol 1450 and pre-melted Gelucire.RTM. 44 / 14 and mixed at about 65.degree. C. to dissolve the drug and give a solution at 4%. by weight.
[0038] The solution was filled into size #1 gray, opaque hard gelatin capsules at 500 mg to provide a dosage form at a strength of 20 mg of the taxane derivative per capsule. Caps were placed on the filled capsule bodies after they were stored at room temperature, for about 30-60 minutes to solidify the filled contents. Capsules were dosed to each of 2 dogs at a dose of approximately 2 mg / kg and plasma samples were taken and analyzed for pharmacokinetic parameters including drug concentrations versus time. Absolute oral bioavailability and coefficient of variation were determined as described above in Example 1.
6 Percentage of Ingredient Amount (mg) Total Composition E Compound Ia 20.0 4.0% PEG 400 120.0 24.0% PEG 1450 120.0 24.0% G...
example 4
[0039] Compound Ia was dissolved at 10% by weight in pre-melted Gelucire 44 / 14 at about 65.degree. C. and the solution was filled into size #1 gray, opaque hard gelatin capsules. Caps were placed on the filled capsule bodies after they were stored at room temperature for about 30-60 minutes to solidify the filled contents. Capsules were dosed to each of 3 dogs at a dose of approximately 3 mg / kg and plasma samples were taken and analyzed for pharmacokinetic parameters including drug concentrations versus time. The AUC's were calculated and used to determine the absolute oral bioavailability relative to Compound Ia administered intravenously to dogs from a PEG 400 solution.
7 Ingredient Amount (mg) Percentage of Total Composition F Compound Ia 30.0 10.0% Gelucire 44 / 14 270.0 90.0% Total 300.0 100.0% Pharmacokinetics F (Oral Bioavailability) 32.7% C.V. (Coefficient of Variation) 2%
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt % | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com