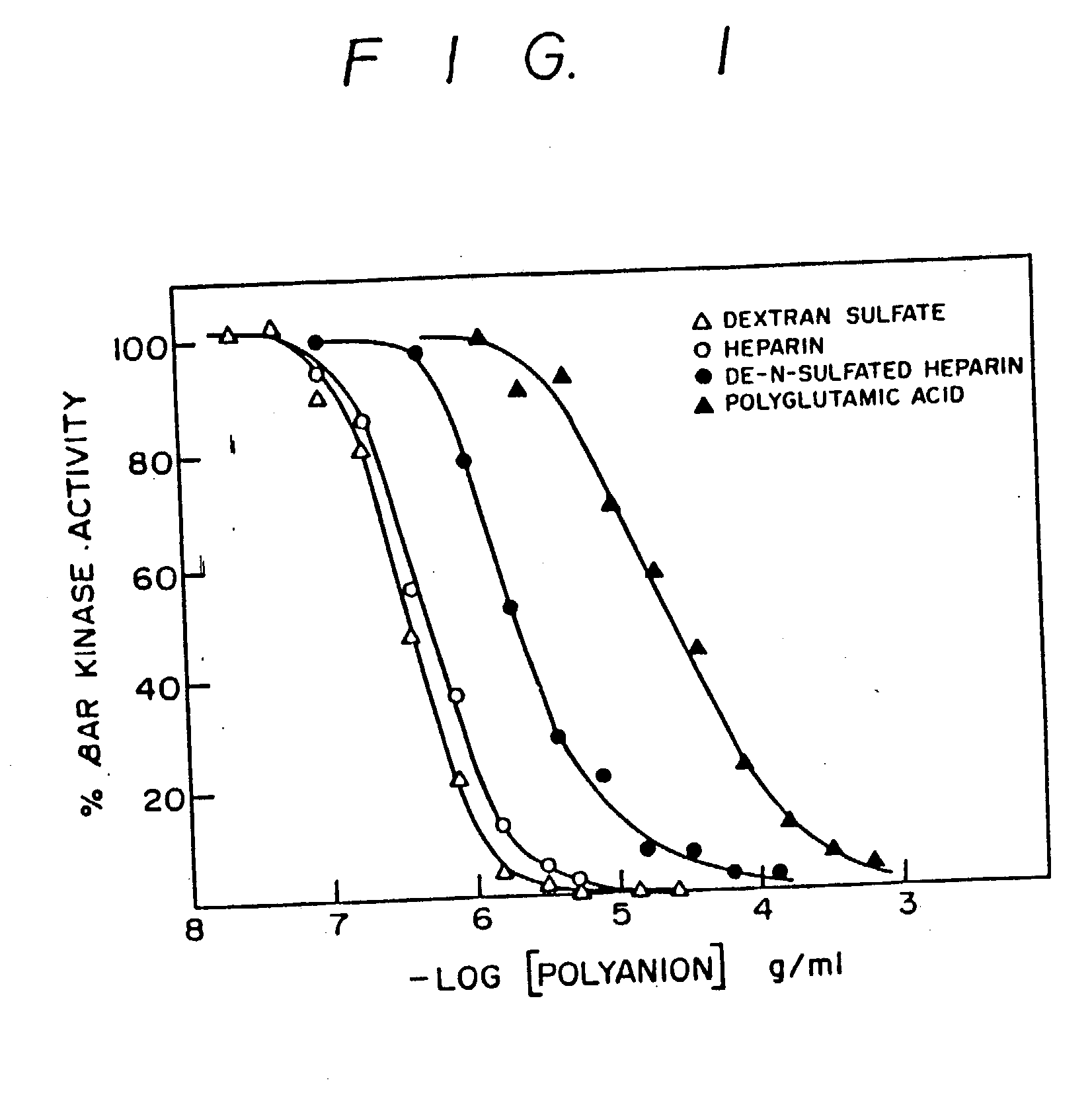
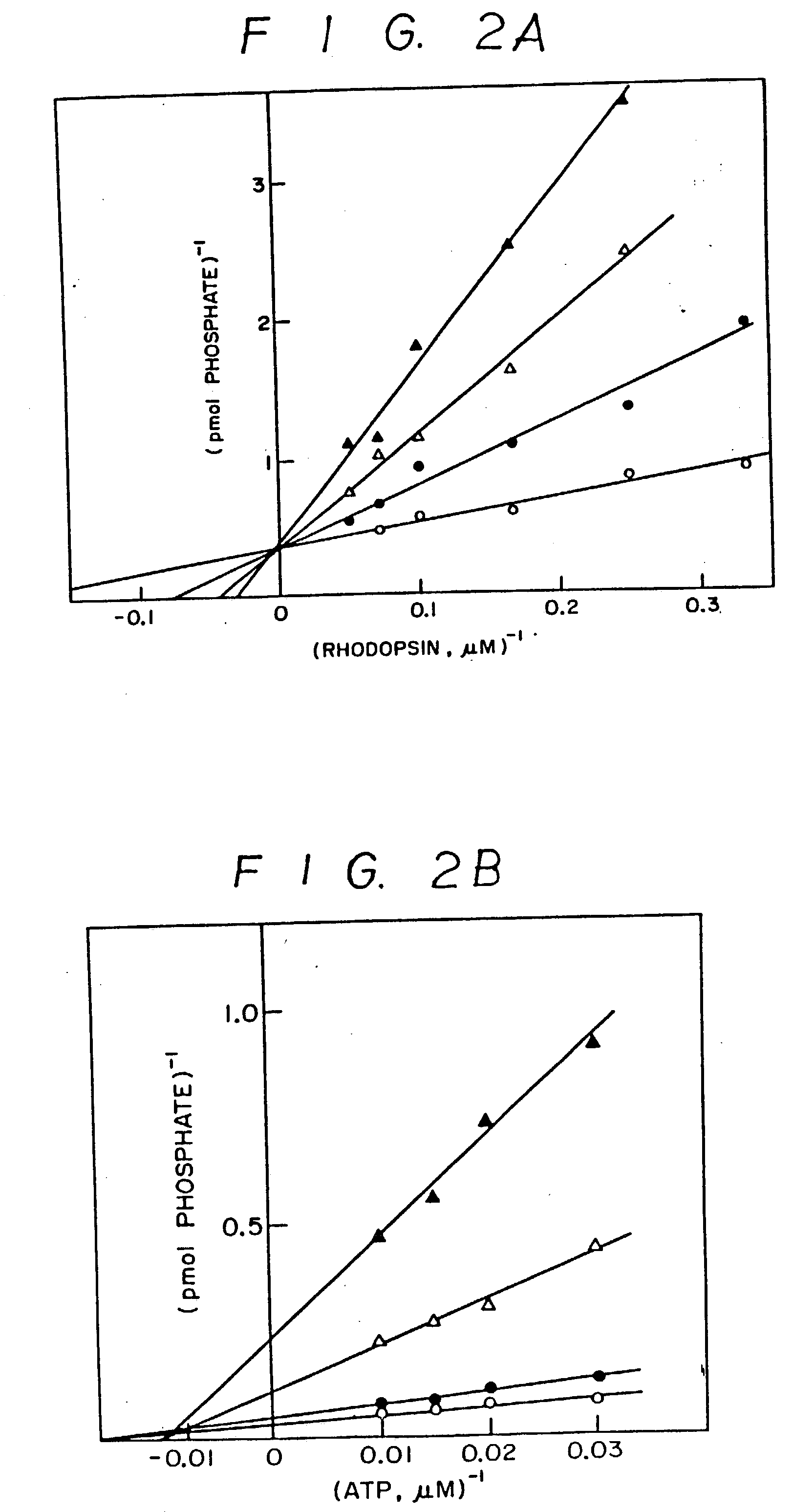
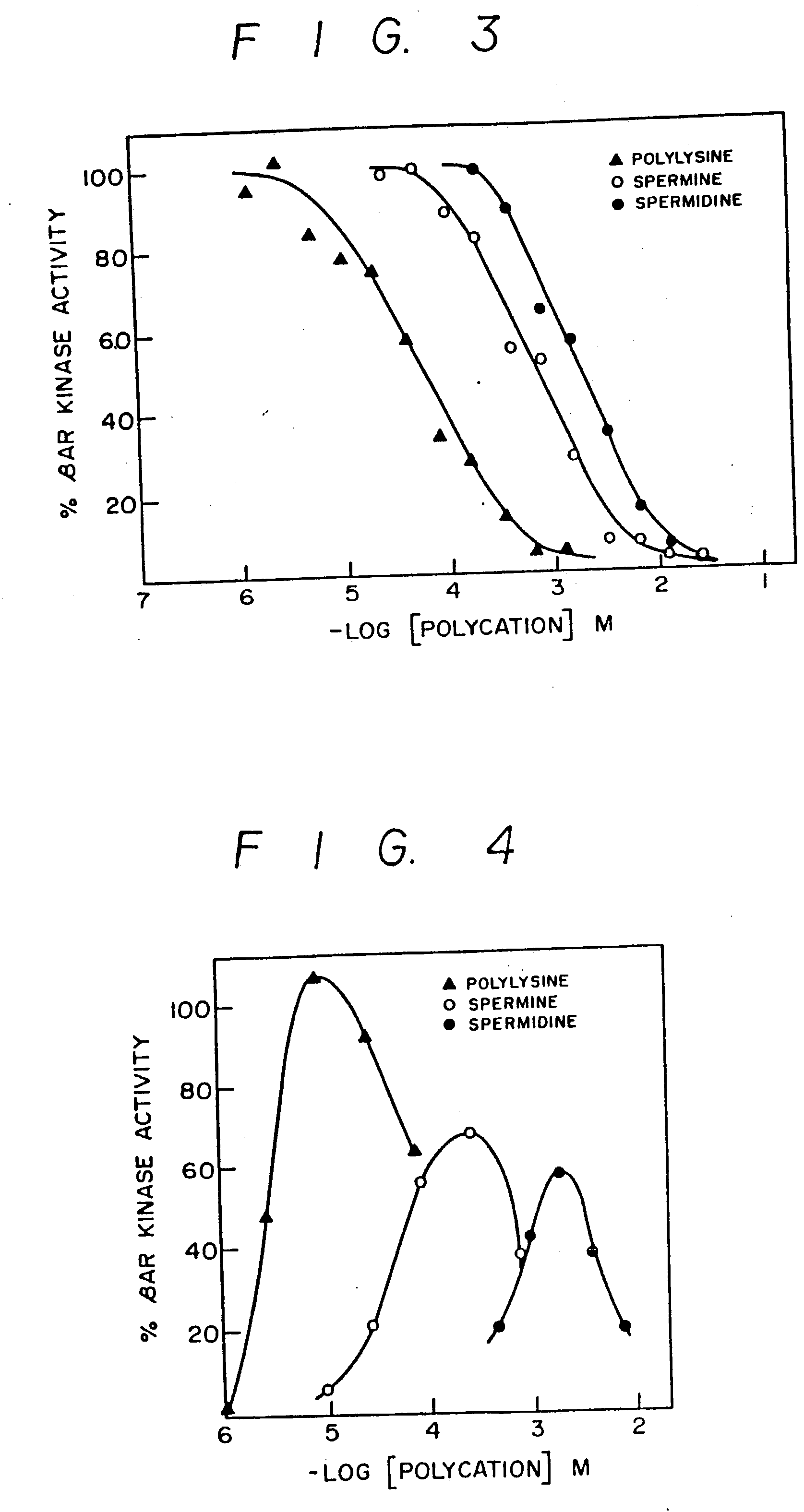
Inhibitors of agonist-specific desensitization
a desensitization and agonist technology, applied in the field of desensitization, can solve the problems of loss of receptor responsiveness to subsequent stimulation, and none of the compounds utilized in these studies directly inhibit the .beta.ar kinas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example ii
Inhibition of .beta.AR Kinase Prevents Rapid Homologous Desensitization of .beta..sub.2-ARs
[0053] [.alpha.-.sup.32P]ATP, [.gamma.-.sup.32P]ATP, [3H]cAMP, .sup.125I-labeled cyanopindolol (.sup.125I-cyanopindolol), and .sup.125I-cyanopindolol diazirine were obtained from New England Nuclear; heparin (H-3125 from porcine mucosa), from Sigma. 1-5(isoquinoline sulfonyl)-2-methylpiperazine (designated H-7), from Calbiochem; and digitonin, from Gallard Schlessinger. The peptide corresponding to residues 1-24 of the heat-stable inhibitor of cAMP-dependent protein kinase [PKI-(1-24) tetracosapeptide: Scott et al Proc. Natl. Acad. Sci. USA (1986) 83:1613-1616] and the .beta..sub.2AR-(57-71) pentadecapeptide (Ala-Ile-Ala-Lys-Phe-Glu-Arg-Leu-Gln-Thr-Val-Thr-Asn-Tyr-Phe; Kobilka et al Proc. Natl. Acad. Sci USA (1987) 84:46-50) and .beta..sub.2AR-(59-69) undecapeptide (Ala-Lys-Phe-Glu-Arg-Leu-Gln-Thr-Val-Thr-Asn) were chemically synthesized.
[0054] Purified .beta..sub.2AR was phosphorylated by pur...
example iii
Synthetic Peptides of the Hamster .beta..sub.2AR as Substrates and Inhibitors of the .beta.AR Kinase
[0070] Peptides were synthesized by tBOC chemistry on an Applied Biosystems 430A Synthesizer. Peptides were deblocked by HF treatment and were purified by reverse phase high performance liquid chromatography on a C18 column using a 0-50% acetonitrile gradient.
[0071] The .beta.2-adrenergic receptor from hamster lung was purified to apparent homogeneity by sequential affinity and high performance liquid chromatography as described (Benovic et al (1984) Biochemistry 23:4510-4518). The purified receptor was reinserted into phosphatidylcholine vesicles as previously described (Cerione et al (1983) Nature 306:562-566). The protein-lipid pellets were resuspended in 20 mM Tris-HCl, pH 7.2, 2 mM EDTA and used as a substrate for the .beta.AR kinase.
[0072] .beta.AR kinase was purified from bovine cerebral cortex by modification of a previously described procedure (Benovic et al (1987) J. Biol. C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com