Implantable electrical cable and method of making
a technology of electrical cables and medical cables, applied in the manufacture of cables/conductors, external electrodes, flexible conductors, etc., can solve the problems of untoward effects on device performance and patient well-being, discomfort for patients, and hostile environment for implanted medical devices and materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The following describes the best mode presently contemplated for carrying out the invention. This description is not to be taken in a limiting sense, but is made merely for describing the general principles of the invention. The scope of the invention should be determined with reference to the claims.
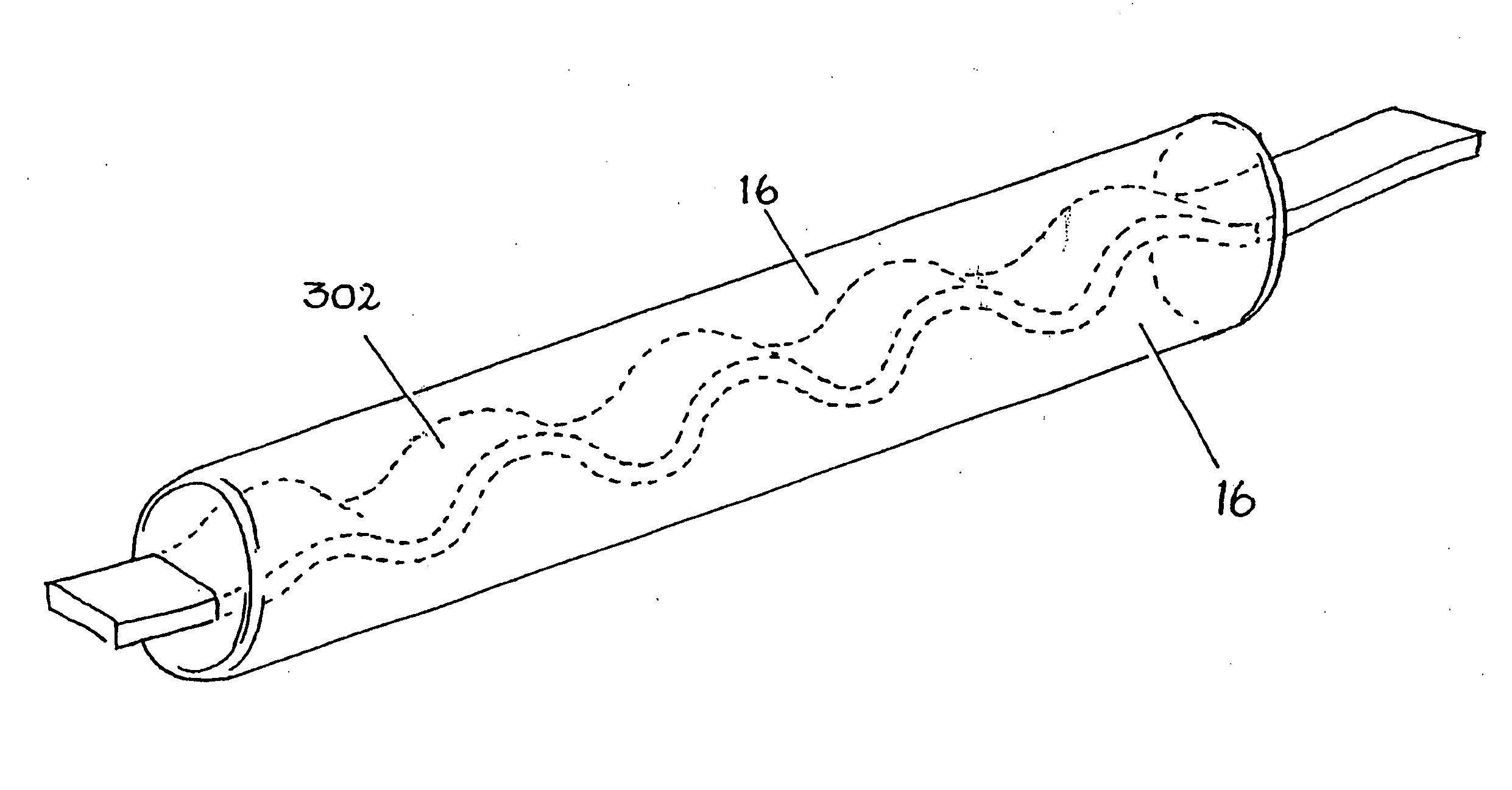
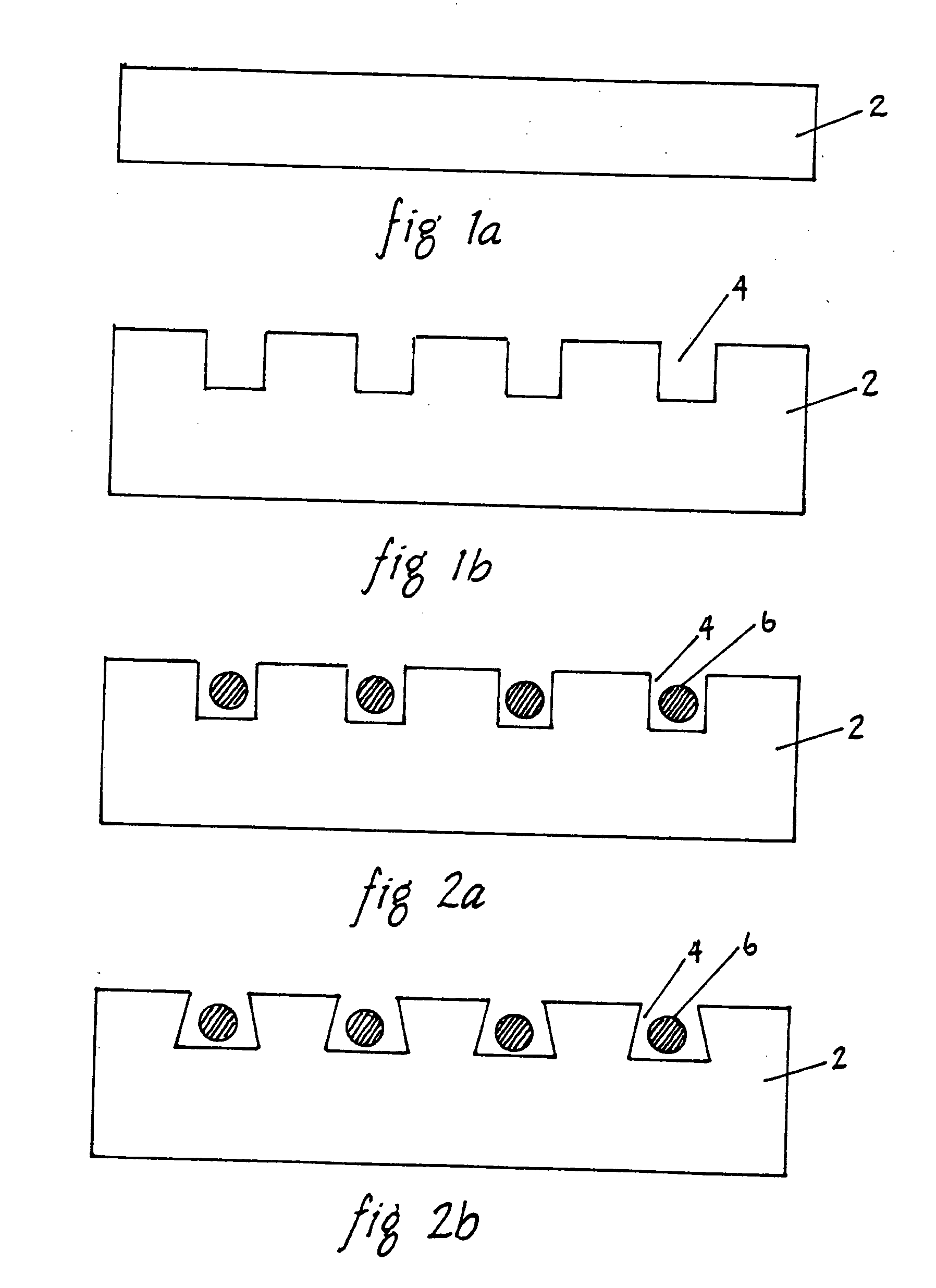
[0031]FIG. 1A is a sectional view of a fluoropolymer film 2 used to manufacture an implantable cable according to the present invention. FEP or PFA film 2 is used in the preferred embodiment of the present invention. However, it is possible to use other melt processable biomaterials such as fluorocarbons PVDF, PCTFE, ECTFE, ETFE, MFA (a copolymer of TFE and PVE), polyethylene's and polypropylenes. The thickness of the film 2 is preferably about 20-100 μm.
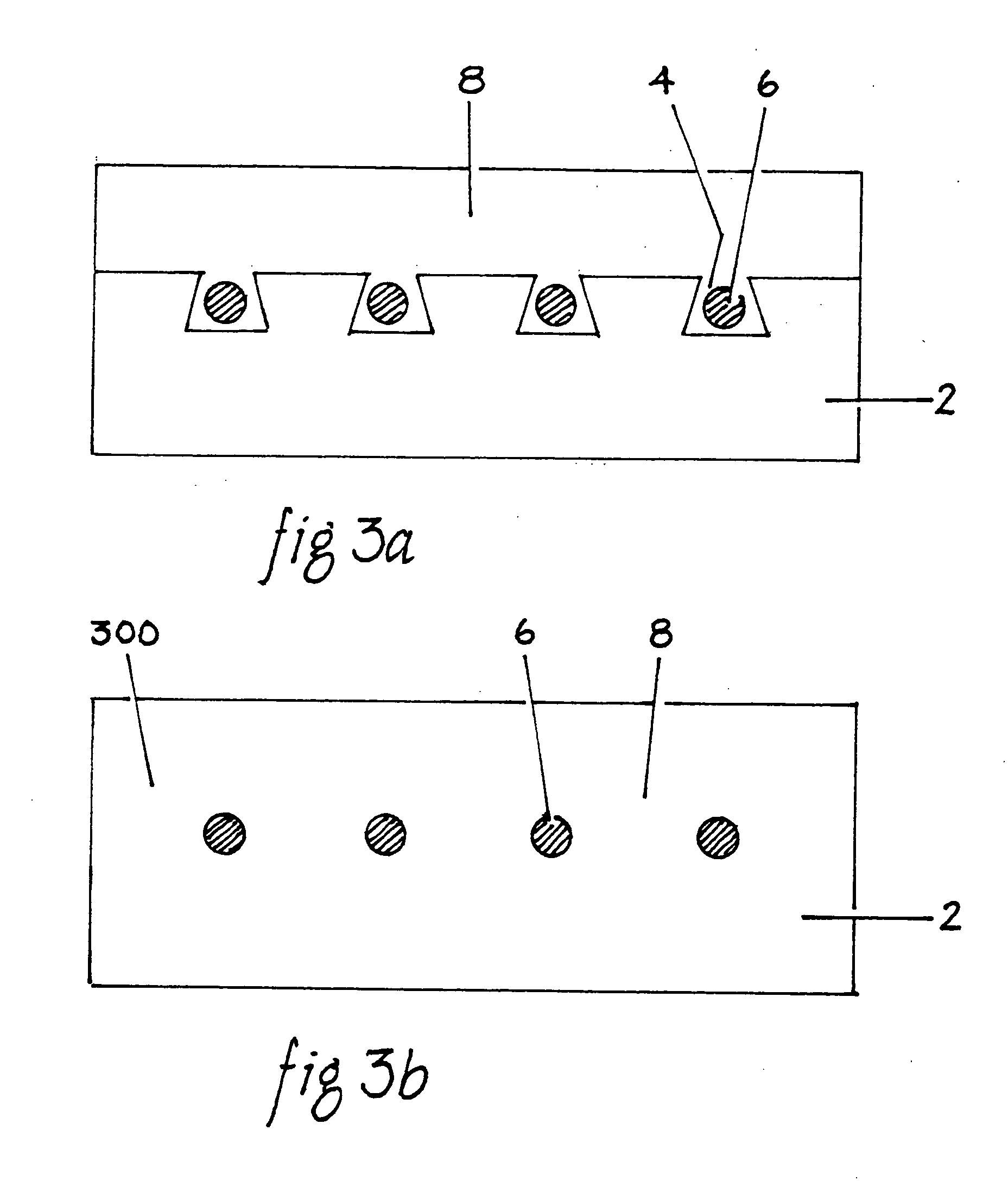
[0032]FIG. 1B is a sectional view of a fluoropolymer film 2 having grooves 4 to manufacture an implantable cable according to the present invention. A plurality of grooves 4 are established within the FEP film 2 through laser c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| biocompatible | aaaaa | aaaaa |
| conductor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com