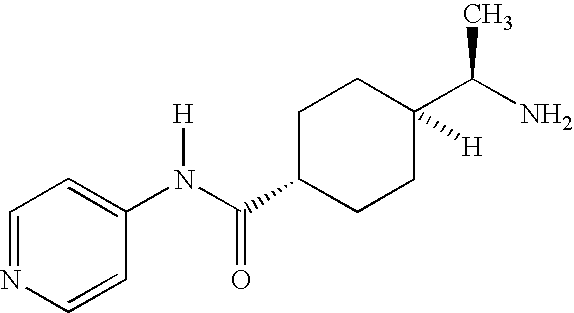
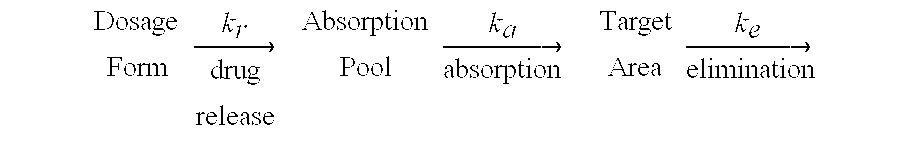As-needed administration of an androgenic agent to enhance female desire and responsiveness
a technology of androgenic agents and as-needed administration, applied in the direction of heterocyclic compound active ingredients, anhydride/acid/halide active ingredients, and active ingredients of elcosanoid active ingredients, can solve the problems of sexual dysfunction, dyspareunia, and affect approximately 40% of women, and achieves high testosterone levels, enhanced female sexual desire and responsiveness, and the effect of increasing the testosterone level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A cream formulation is prepared using testosterone enanthate. The cream includes the following components:
Testosterone enanthate 100 mgBeeswax 2.7 gmCarbopol ® 934 q.s.100.0 gm
Mixing is conducted with tile and spatula until a homogeneous cream mixture is obtained having testosterone enanthate uniformly dispersed throughout the formulation.
example 2
The procedure of Example 1 is repeated except that the following components are used:
Testosterone propionate 100 mgPolyethylene glycol 40037.5 gm1,2,6-hexanetriol20.0 gmPolyethylene glycol 4000 q.s.100.0 gm
A homogenous cream mixture is obtained.
example 3
The procedure of Example 1 is repeated except that the following components are used:
Testosterone cypionate 100 mgPolyethylene glycol 40037.0 gmPolyethylene glycol 400 monostearate26.0 gmPolyethylene glycol 4000 q.s.100.0 gm
A homogenous cream mixture is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| muscular tension | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com