Detection of endometrial pathology
a technology of endometrial pathology and detection method, which is applied in the direction of biological material analysis, material analysis, instruments, etc., can solve the problems of low specificity, no routine screening test of practical utility, and carry the risk of uterine perforation or contamination of the cavity, so as to increase the predictive power of the screening assay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0080] The present invention is illustrated by the following examples. It is to be understood that the particular examples, materials, amounts, and procedures are to be interpreted broadly in accordance with the scope and spirit of the invention as set forth herein.
example i
Identification of Peaks Associated With Endometrial Cancer
Summary
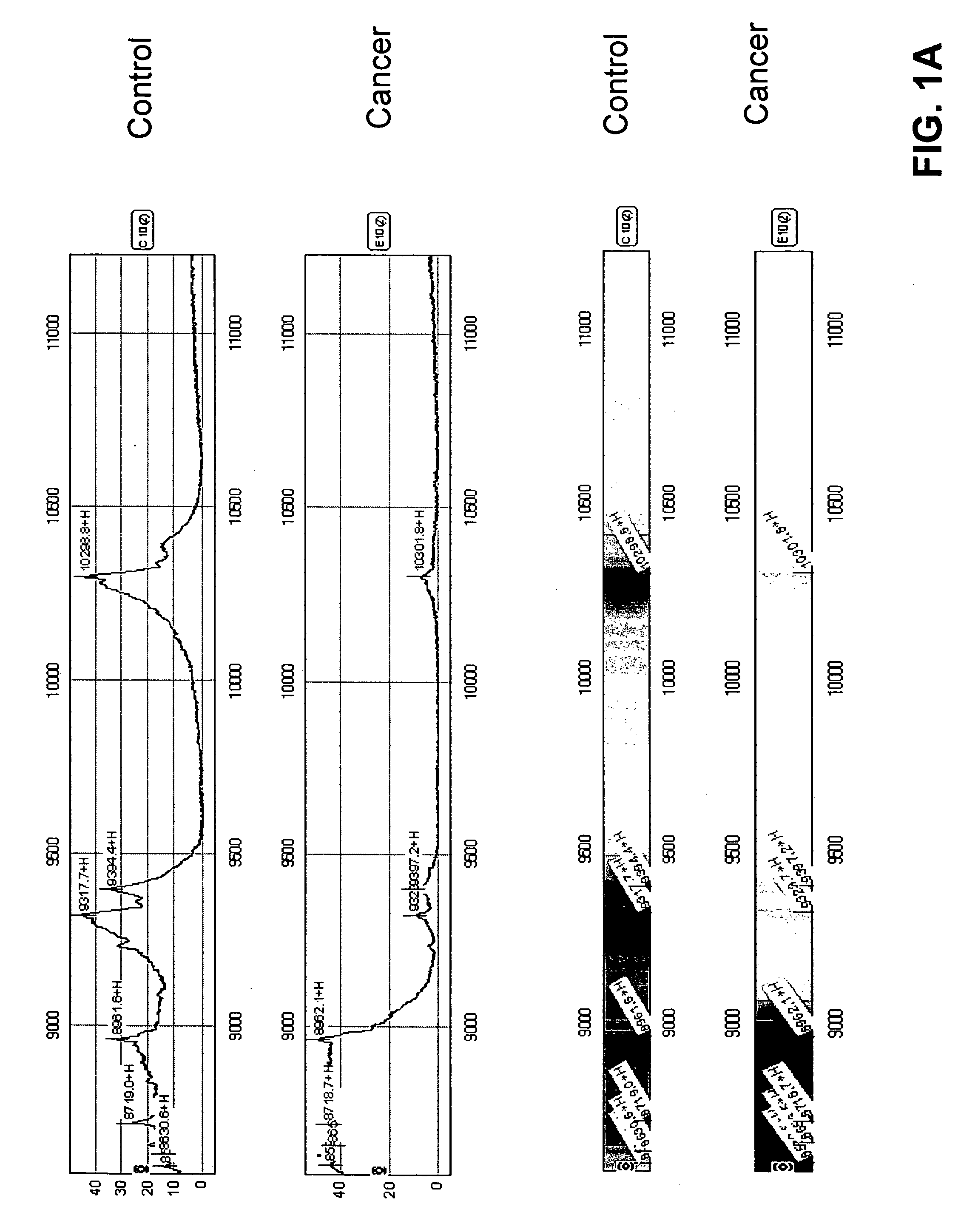
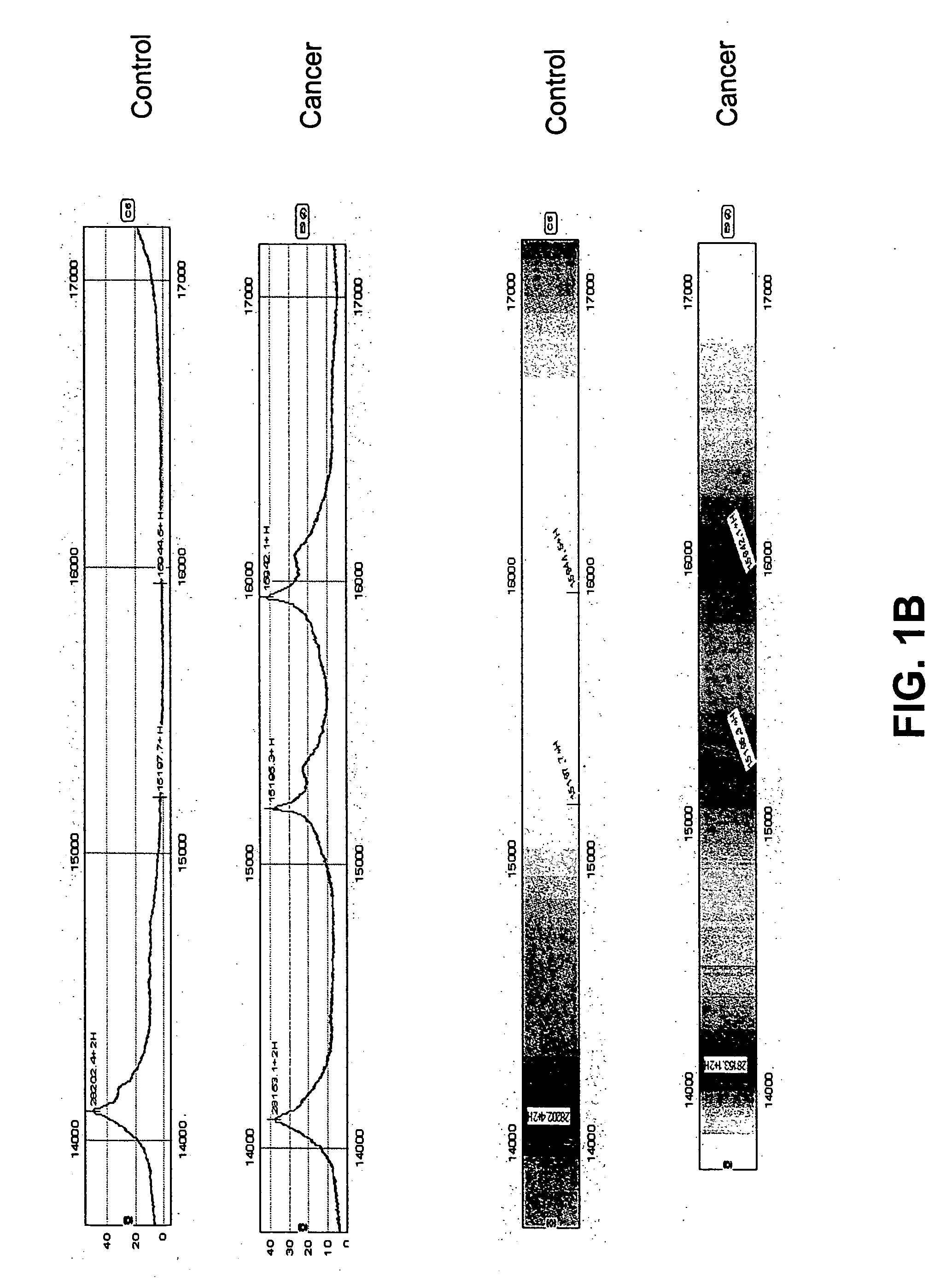
[0081] Mass spectrometry data were collected from serum samples from women with endometrial cancer and from controls. A candidate protein expression profile was identified that appears to distinguish early stage endometrial carcinoma from healthy controls. Peptide spectra were used to create algorithms with high sensitivity and high specificity in discriminating endometrial cancer.
Methods
[0082] Serum was collected in serum separator tubes. It is envisioned that in future work serum will be collected from all patients at diagnosis, at specific times during therapy, 6 months post-therapy, and at recurrence. At collection, the serum is spun in a low speed centrifuge at 1500 rpm for 3 minutes, and the serum is aliquoted into 1 ml cryovials and immediately frozen in liquid nitrogen. Samples will be forwarded to a central tissue collection facility for storage. At the laboratory, samples are thawed, a protease inhibito...
example ii
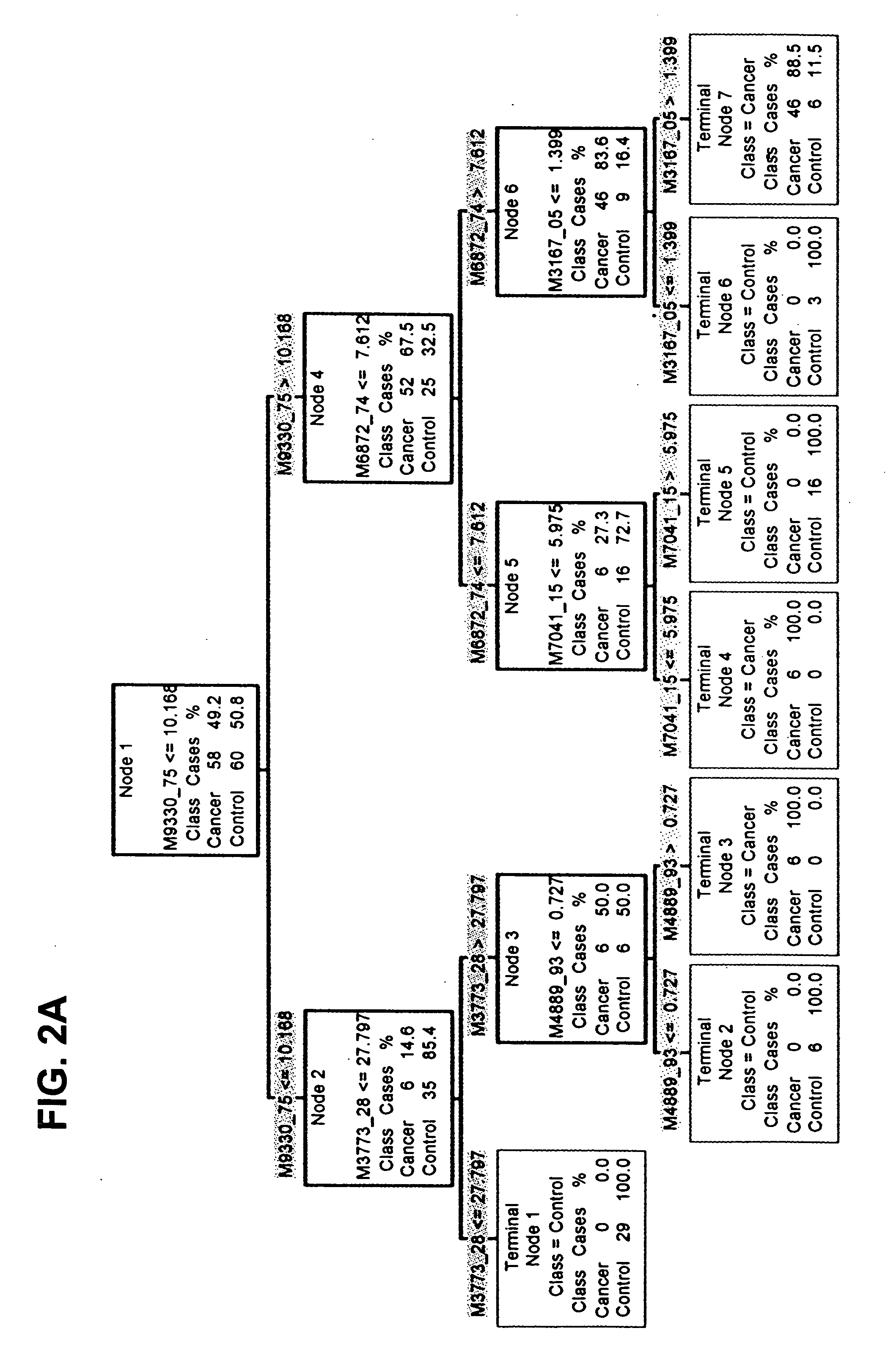
Identification of Particular Polypeptide Biomarkers
[0119] In Example I, whole serum samples were fractionated using Q Ceramic Hyper DF sorbent columns. Any potential discriminatory biomarkers discovered will have already been assigned to a known pH fraction on the IMAC 30 ProteinChip Array surface. Using this knowledge, a marker can be first purified using Q HyperD F column chromatography, then the pH fraction of interest can be purified to enrich for the biomarker. The fraction containing the marker is purified through a second chromatography step using IMAC HyperCel Spin Columns (Ciphergen, Fremont Calif.), which have a matched chemistry to IMAC 30. The marker is additionally purified, concentrated and desalted using a reversed phase step. Finally the protein of interest is purified using one dimensional SDS PAGE.
[0120] Following in-gel digestion, protein identification can be attempted by MALDI Time of Flight Mass Spectrometry (MALDI-TOF) using an AP Biosystems Voyager Elite in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com