Non-compliant medical balloon having a longitudinal fiber layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
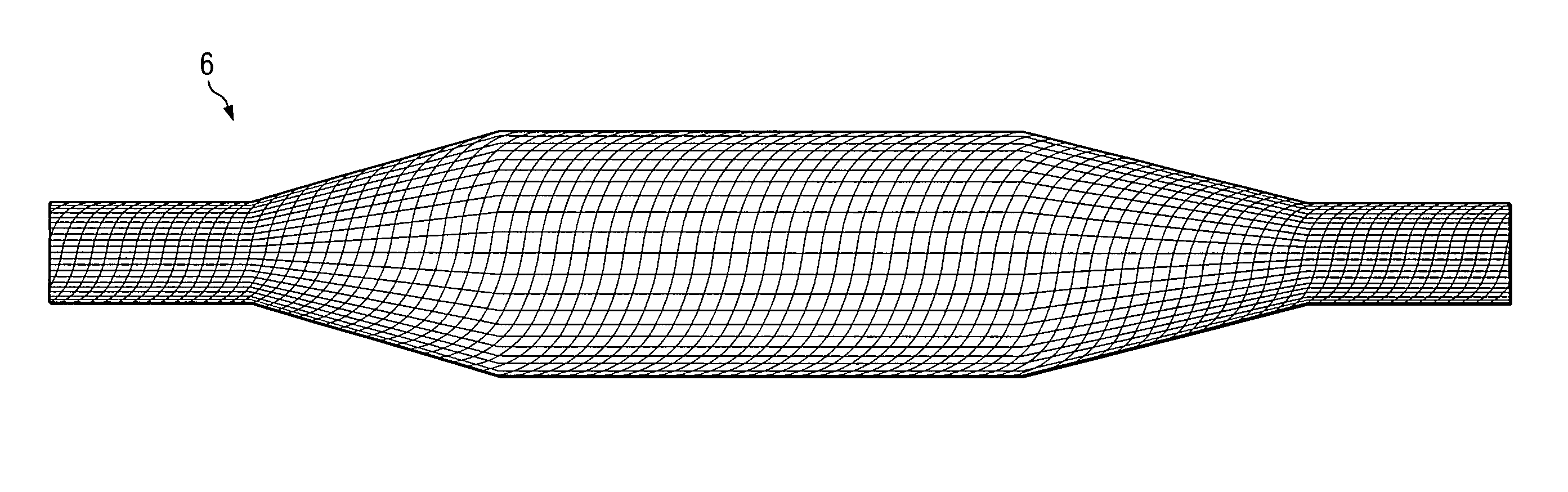
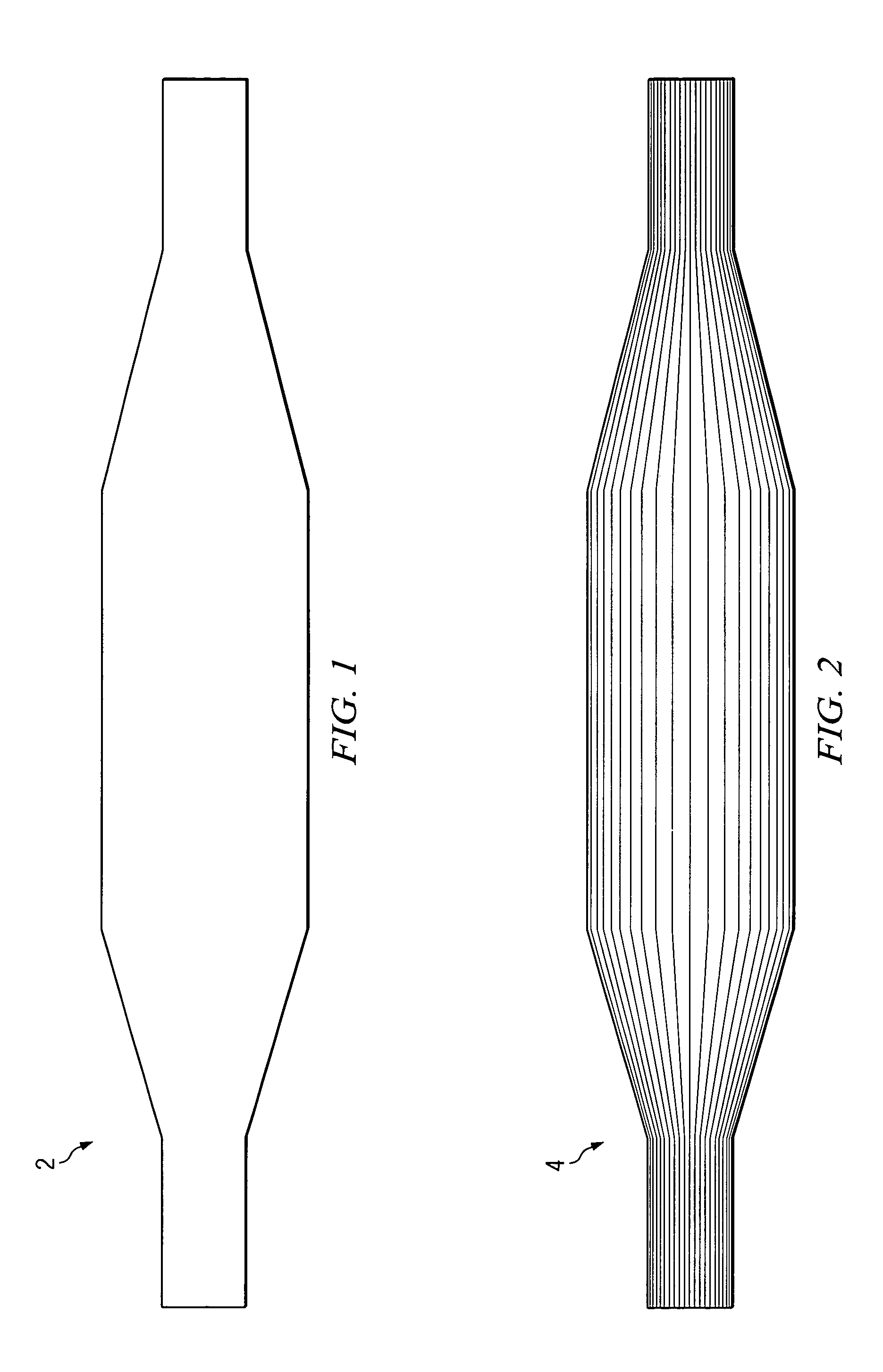
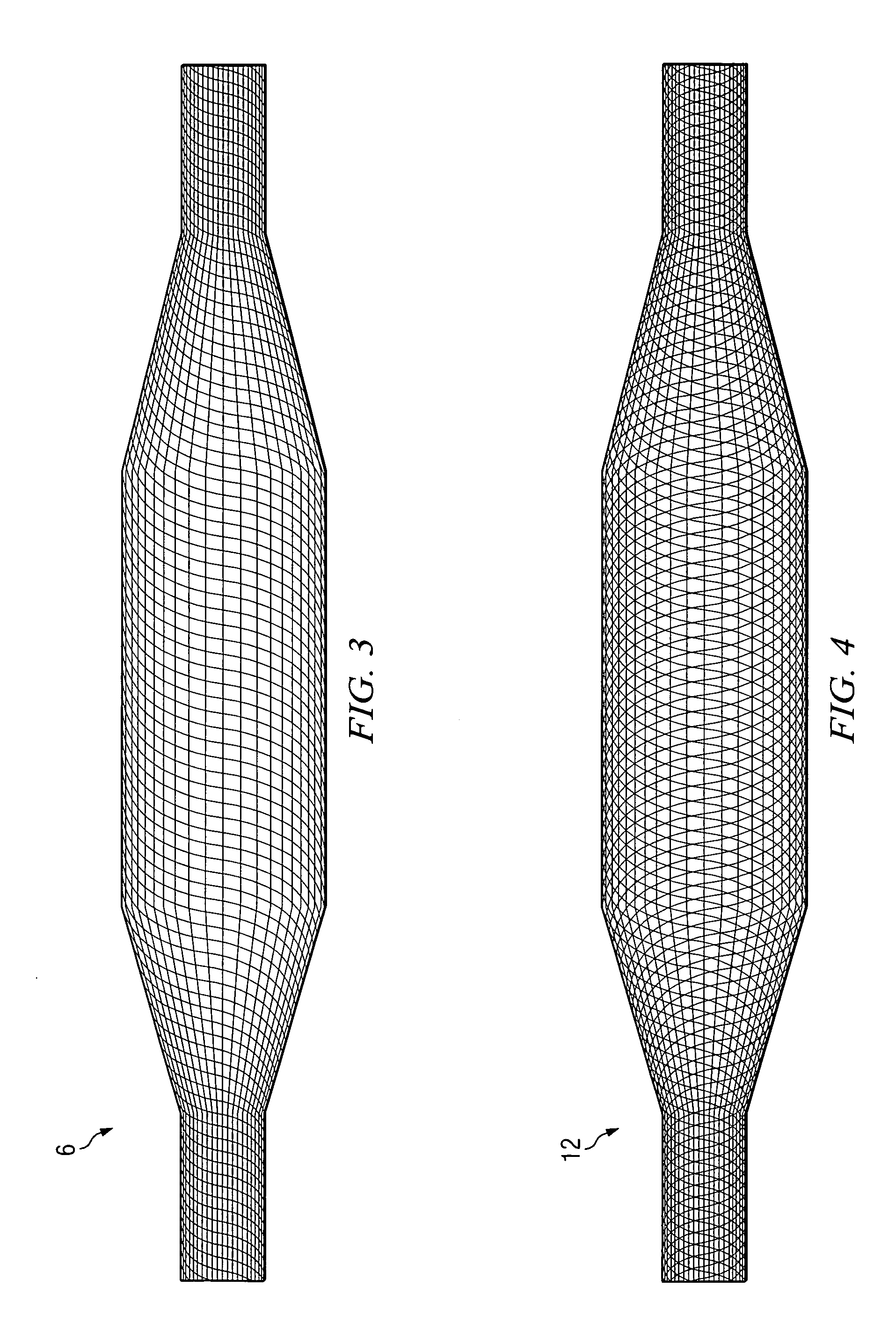
[0027] An angioplasty balloon, as shown in FIG. 1, having a wall thickness of 0.0008 inch is inflated to about 100 psi, and the two open ends of the balloon are closed off. The inflation pressure maintains the shape (geometry) of the balloon in an inflated profile during the construction of the composite balloon. The balloon is a blow-molded balloon of highly oriented polyethylene terephthalate (PET). To the inflated balloon is applied a very thin coat of 3M-75 adhesive to hold the fibers sufficiently to prevent them from slipping out of position after placement on the balloon.
[0028] Kevlar® fibers are placed, by hand, along the length of the balloon as shown in FIG. 2 to provide the primary wind. Each of the fibers is substantially equal in length to the length of the long axis of the balloon. Twenty-four fibers are used, substantially equally spaced from each other. The fiber used for the primary wind has a thickness of 0.0006 inch.
[0029] Next, a hoop wind of Kevlar® fiber is ap...
example 2
[0032] The procedure of Example 1 was repeated with the exception that Vectran® fiber, having a thickness of 0.0005 inch is used in place of the Kevlar® fiber. The resulting composite balloon is axially and radially non-compliant at very high working pressures. The balloon has very high tensile strength and abrasion and puncture resistance.
[0033] Vectran® is a high strength fiber available from Hoechst-Celanese, Charlotte, N.C.
example 3
[0034] A mandrel in the shape of a balloon as shown in FIG. 1 is made of a water-soluble wax. The wax mandrel is coated with a very thin layer (0.0002 inch) of Texin® 5265 polyurethane. After curing, adhesive and Vectran® fibers are applied, following the procedure of Example 1. Next, several coats of Texin® 5265 polyurethane as applied in Example 1. The wax is then exhausted by dissolving in hot water to give a non-compliant, very high strength, abrasion-resistant, composite fiber-reinforced balloon.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com