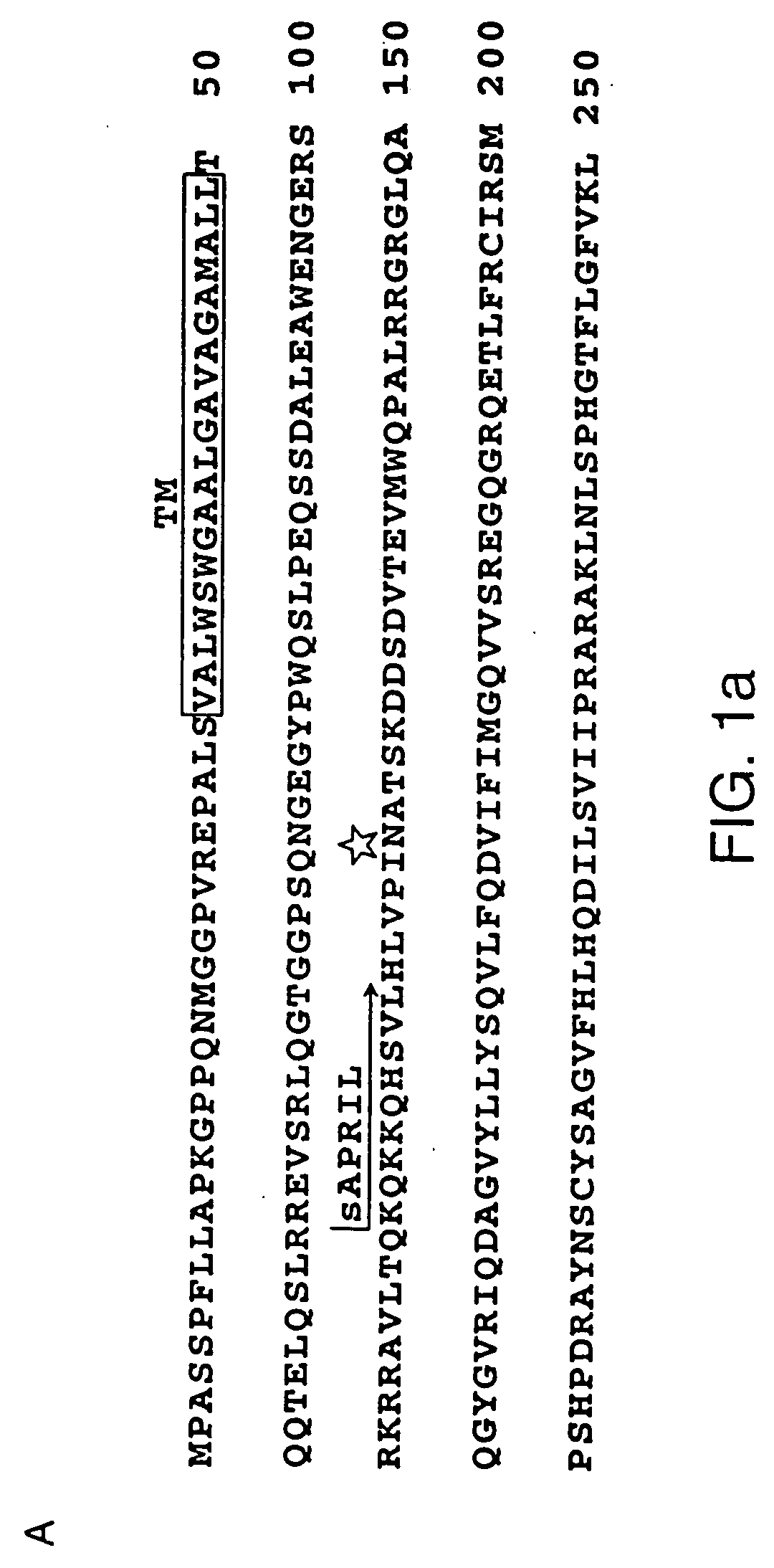
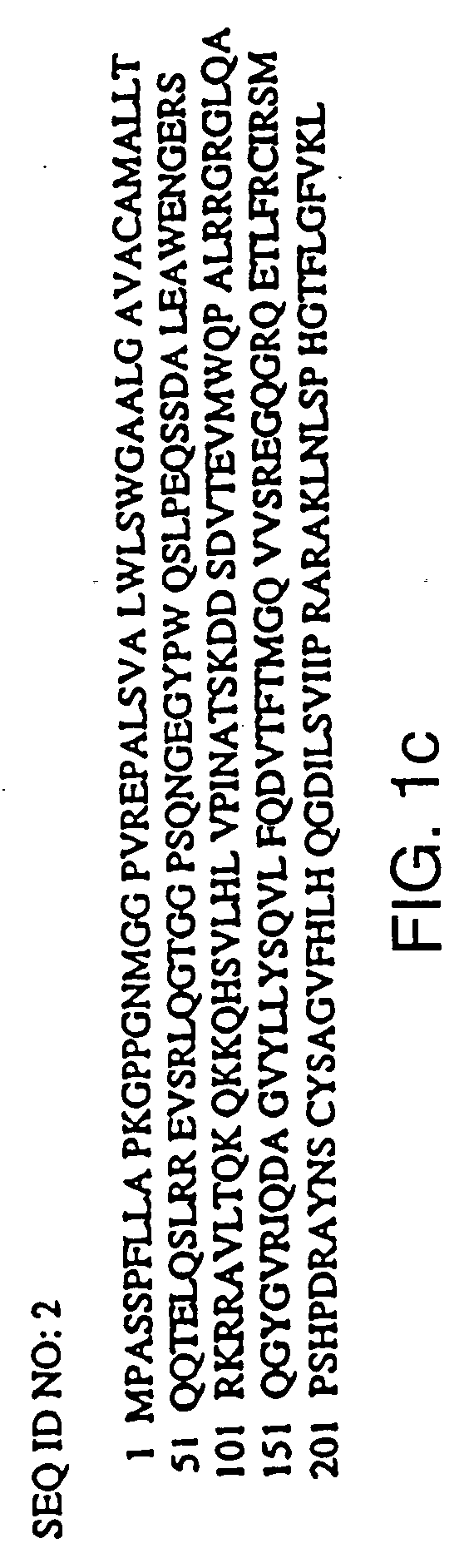
Heterologous polypeptide of the TNF family
a tumor necrosis factor and heteromeric ligand technology, applied in the field of apbf, can solve the problems of complex ligand/receptor interaction, increase, etc., and achieve the effect of stimulating b-cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0065] This example describes the detection of APRIL and BAFF heteromers by immunoprecipitation following co-transfection into mammalian cells.
Methods:
[0066] The plasmids encoding FLAG-tagged human soluble APRIL, beginning with residue A105 (LT033) or K110 (PL448), soluble FLAG-tagged human TWEAK beginning at A106 (PS288) or soluble FLAG-tagged human EDA beginning at A242 (PS548) or empty vector (CH269) were co-transfected with a full-length human BAFF construct (PS544) into 293T cells using lipofectamine (Life Technologies). At 48 hrs. post-transfection, conditioned media was collected and used for immunoprecipitation experiments. The immunoprecipitation samples contained 200 μl of conditioned media, 5 μg / ml of the anti-FLAG antibody M2 (Sigma) and 800 μl of DMEM containing 10% FCS, glutamine, Pen-Strep, G418 and sodium azide and were incubated at 4° C. for 1 hour, with agitation. Then, 30 μl of ProteinA-Sepharose beads (Pharmacia) was added to the samples and the mixture was in...
example 2
[0071] This example describes the detection of APBF heteromers by immunoprecipitation following co-transfection of two soluble constructs into mammalian cells.
Methods:
[0072] Plasmids encoding the following human soluble TNF family ligands were constructed with the indicated N-terminal epitope tags beginning at the ligand amino acid residue indicated in a PCR3 based mammalian cell expression vector: FLAG-APRIL, beginning with residue A105 (plasmid #LT033) or H115 (plasmid #LT038), FLAG-TWEAK A106 (plasmid #PS288), myc-APRIL A105 (plasmid #JST557), and myc-BAFF Q136 (plasmid #JST556). Various constructs encoding FLAG-tagged ligands, full length murine APRIL(plasmid #LT022), or empty vector control (plasmid #CH269) were each co-transfected with the myc-BAFF Q136 construct into 293T cells using lipofectamine (Life Technologies, Gaithersburg, Md.). At 48 hrs. post-transfection, conditioned media was collected and used for immunoprecipitation experiments. The immunoprecipitation sample...
example 3
Production and isolation of APBF by Affinity Methods
[0077] Plasmids encoding the following human soluble TNF family ligands were constructed with N-terminal FLAG or 6×His epitope tags beginning at the amino acid residue indicated in a PCR3 based mammalian cell expression vector: FLAG-APRIL, beginning with residue A87 (plasmid Lf133) and RGS(H)6-BAFF Q134. These plasmids are then co-transfected into 293T cells using lipofectamine (Life Technologies, Gaithersburg, Md.) and at 48 hrs. post-transfection, conditioned media is collected. The conditioned media is dialyzed against 50 mM NaH2PO4, pH8.0; 300 mM NaCl; 10 mM imidazole and run over a Ni—NTA Superflow column (Qiagen, Valencia, Calif.). Homomers and heteromers containing the 6×His tagged BAFF subunit bind to the Ni column; homomeric FLAG-APRIL molecules flow through. The column is washed with 50 mM NaH2PO4, pH8.0; 300 mM NaCl; 20 mM imidazole with 5-10 column volumes. The column is eluted with 5 column volumes with 50 mM NaH2PO4,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com