Medicament storage and delivery devices
a technology of storage and delivery device and medicine, which is applied in the direction of microcapsules, pharmaceutical packaging, packaging, etc., can solve the problems of adversely affecting the release, stability and bioavailability of active ingredients, and the dispensing and administration of solid dosage forms are not without associated problems and drawbacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
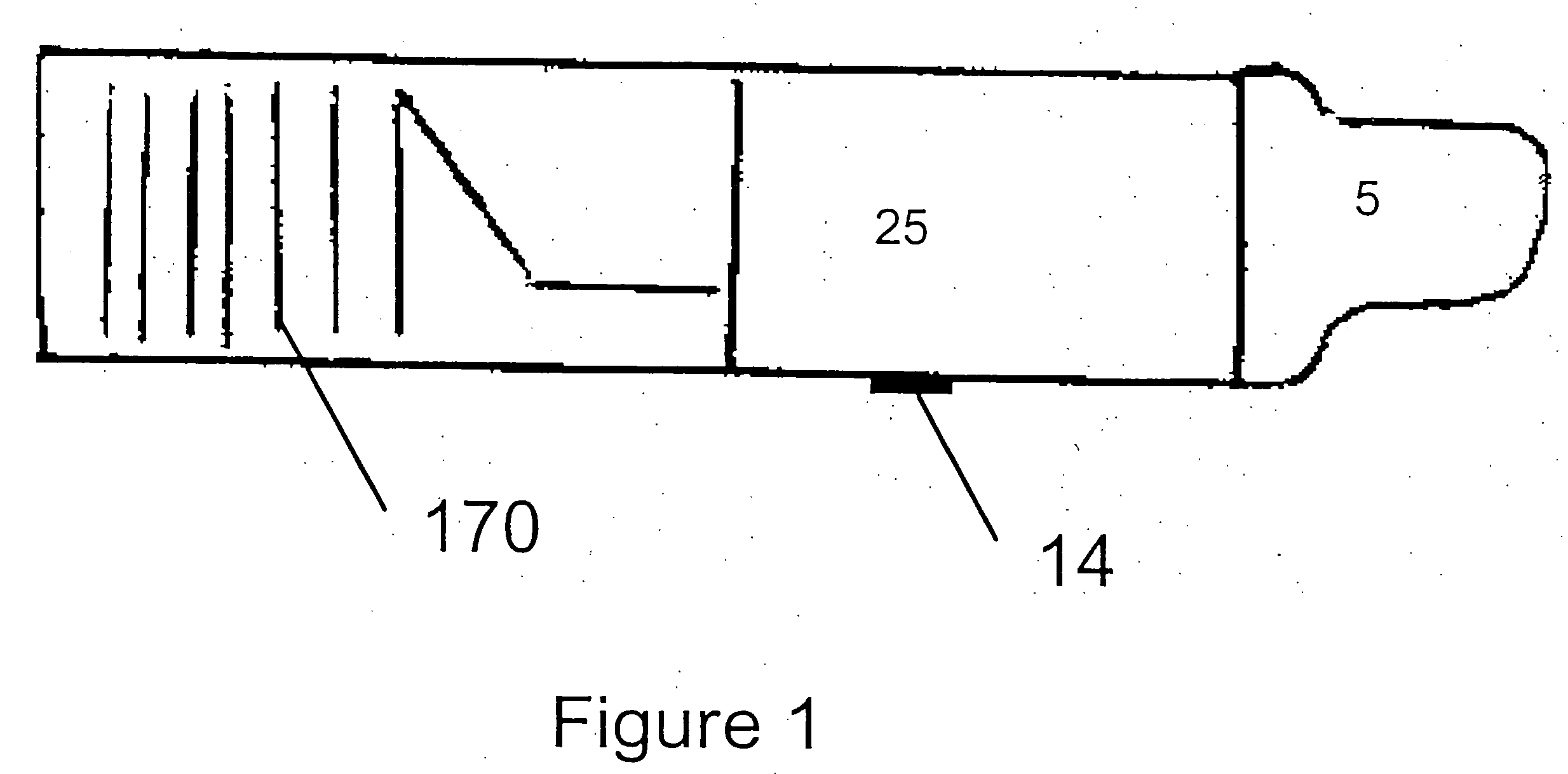
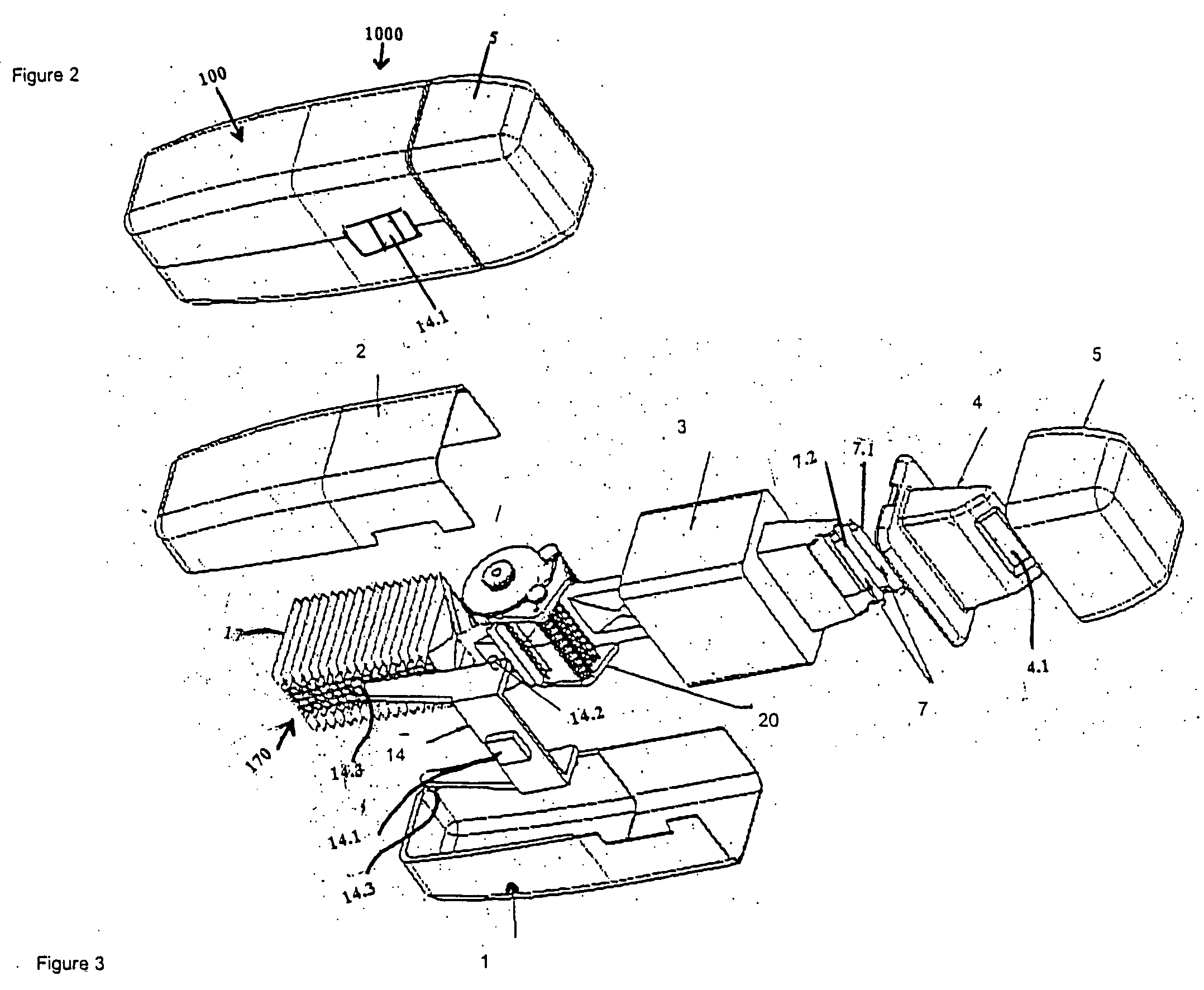
[0066]FIG. 2 shows a perspective view of a drug delivery device 1000 in accordance with the present invention, and FIG. 3 shows an exploded perspective view of FIG. 2. Further details of the device 1000 are shown in FIGS. 4(a,b). Referring to FIG. 3, a drug delivery device 1000 includes a housing 100 comprised of a first body portion 1 and a second body portion 2. The sachet pack 170 described above is shown in further detail in FIG. 4(b). The sachet pack 170 is comprised of a plurality of individual sachets 17, and is stored within the housing 100. The sachet pack 170 is a continuous sheet which is comprised of a pair of strips 1701, 1702 which are adhered to each other and which enclose a medicament.
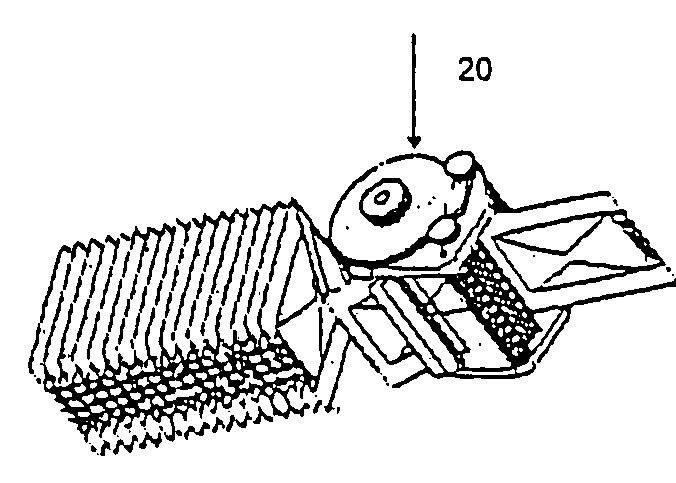
[0067] The device 1000 includes a chassis 3 having a pair of lead rollers 7.1, 7.2 (collectively, lead rollers 7), wherein roller 7.1 is offset forward and above with respect to roller 7.2 as shown in FIG. 3. A sachet drive mechanism 20, which is partially enclosed within a hollow inte...
second embodiment
[0077] the present invention (device 1000′) is illustrated in FIGS. 6-10, with similar components bearing similar reference numerals to FIGS. 1-5.
[0078] Referring to FIGS. 10(a)-10(d), in use, a patient moves cover 5′ first in a direction 21 (FIGS. 6 and 10(b)), and then in a direction 22 (FIGS. 6 and 10(c)) to expose the mouthpiece 4. Preferably, the cover 5′ can be secured on the rear of the housing 100′ as shown in FIG. 10(d). As the cover 5′ moves in the direction 22 (clockwise from the perspective in FIG. 6 and counterclockwise from the perspective in FIG. 10(b)), lever 5.1 turns cover hinge 9.21 clockwise (from the perspective of FIG. 6), thereby tensioning torsion spring 9.22, which, in turn, applies a clockwise force to gear 9.23.
[0079] As shown in FIGS. 6 and 9, actuator 14′ includes an index button 14.1′ which extends through opening 102 in housing 100′. Actuator 14′ further includes an arm 14.2 having a protrusion 14.3. Arm 14.2 extends between gear 9.1 and housing part ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean diameter | aaaaa | aaaaa |
| mean diameter | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com