Stereoselective synthesis of certain trifluoromethyl-substituted alcohols
a trifluoromethyl and alcohol technology, applied in the field of selective synthesis of certain trifluoromethylsubstituted alcohols, can solve the problems of chiral hplc and other enantiomer separation methods, which are generally unsuitable for large-scale preparation of single enantiomer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
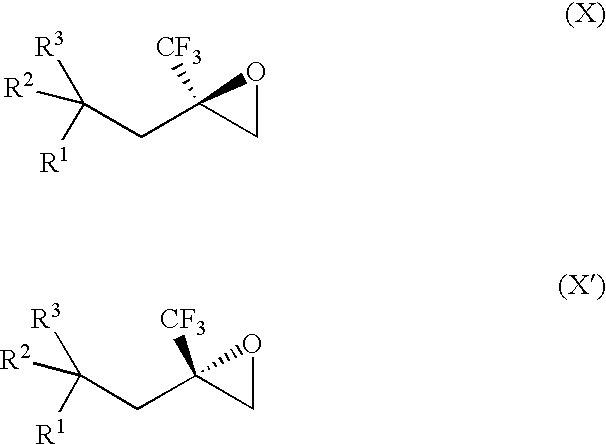
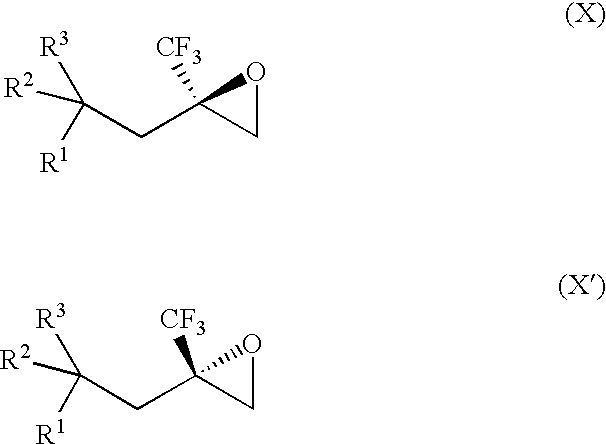
[0073] The invention provides processes for making compounds of Formula (X) or (X′). In all schemes, unless specified otherwise, R1 to R3 in the formulas below have the meanings of R1 to R3 in the Summary of the Invention section. For synthesis of intermediates, see the synthetic procedures disclosed in U.S. Patent Application Publication Nos. 2004 / 0023999 and 2004 / 0162321, which are each incorporated herein by reference in their entireties. Other intermediates used in the preparation of compounds of the invention are either commercially available or readily prepared by methods known to those skilled in the art.
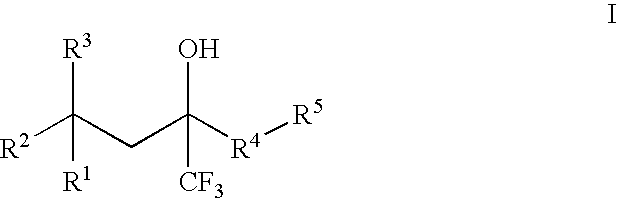
[0074] The epoxide of Formula (II) is a key intermediate in the synthesis of certain racemic compounds of Formula (I), as described in U.S. Patent Application Publication No. 2004 / 0162321, which is hereby incorporated by reference. Treatment of the epoxide of Formula (II) with the nucleophile R5H, in the presence of base opens the epoxide to provide racemic (I) as shown belo...
example 1
Synthesis of (R)-2-[2-(5-Fluoro-2-methoxyphenyl)-2-methylpropyl]-2-trifluoromethyloxirane
[0080]
[0081] To a suspension of (R)-(+)-methyl p-tolylsulfoxide (28.2 g, 183 mmol) in 200 mL of anhydrous THF at −78° C. was added lithium diisopropylamide (LDA) mono(tetrahydrofuran), 1.5 M solution in cyclohexane (122 mL, 183 mmol) over 30 minutes. The resulting clear yellow solution was stirred for an additional 15 minutes. 1,1,1-Trifluoro-4-(5-fluoro-2-methoxyphenyl)-4-methylpentan-2-one (46.3 g, 166 mmol) dissolved in 125 mL of THF was then added via cannula over 30 minutes. After 1.5 hours at −78° C., the reaction mixture was quenched with 600 mL of water and extracted first with a 600 mL portion of EtOAc and then a 400 mL portion of EtOAc. The combined organic phases were washed with saturated aqueous sodium bicarbonate (NaHCO3) solution, washed with brine, dried over sodium sulfate (Na2SO4), filtered, and concentrated in vacuo. Purification by column chromatography with silica gel (elut...
example 2
Synthesis of (R)-2-[2-(5-Fluoro-2-methylphenyl)-2-methylpropyl]-2-trifluoromethyloxirane
[0084]
[0085] To a suspension of (R)-(+)-methyl p-tolylsulfoxide (1.00 g, 6.48 mmol) in 10 mL of anhydrous THF at −78° C. was added LDA mono(tetrahydrofuran), 1.5 M solution in cyclohexane (4.32 mL, 6.48 mmol) over 5 minutes. The resulting clear yellow solution was stirred for an additional 15 minutes. 1,1,1-Trifluoro-4-(5-fluoro-2-methylphenyl)-4-methylpentan-2-one (1.55 g, 5.90 mmol) was then added via cannula with the aid of 4 mL of THF over 5 minutes. After 1 hour at −78° C., the reaction mixture was quenched with 50 mL of water and extracted with three 50 mL portions of EtOAc. The combined organic phases were washed with saturated aqueous sodium bicarbonate solution, brine, dried over sodium sulfate, filtered, and concentrated in vacuo. Purification by column chromatography with silica gel (eluted with 15%-25% EtOAc / hexanes) afforded sequentially (S)-1,1,1-trifluoro-4-(5-fluoro-2-methylpheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com