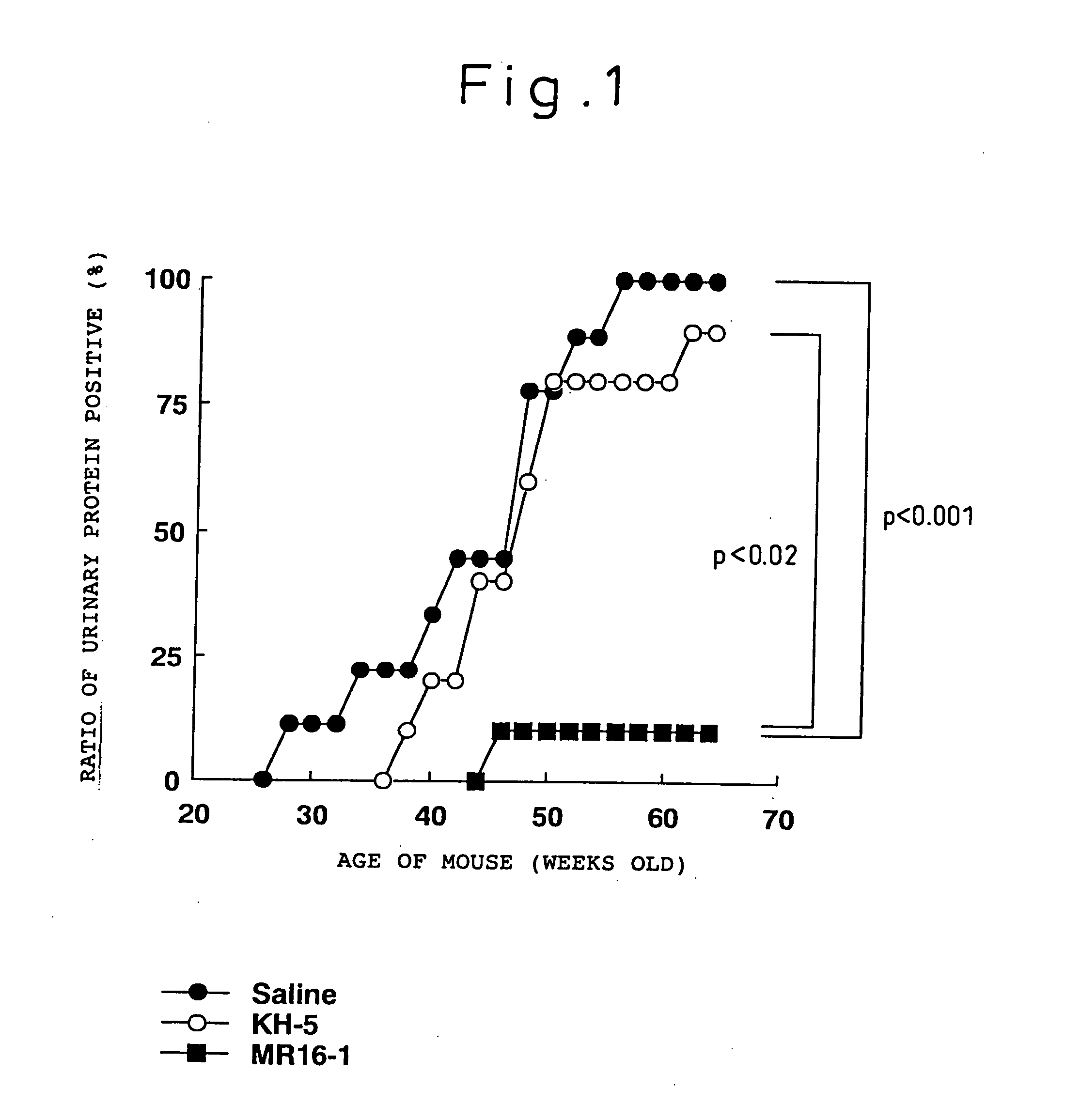
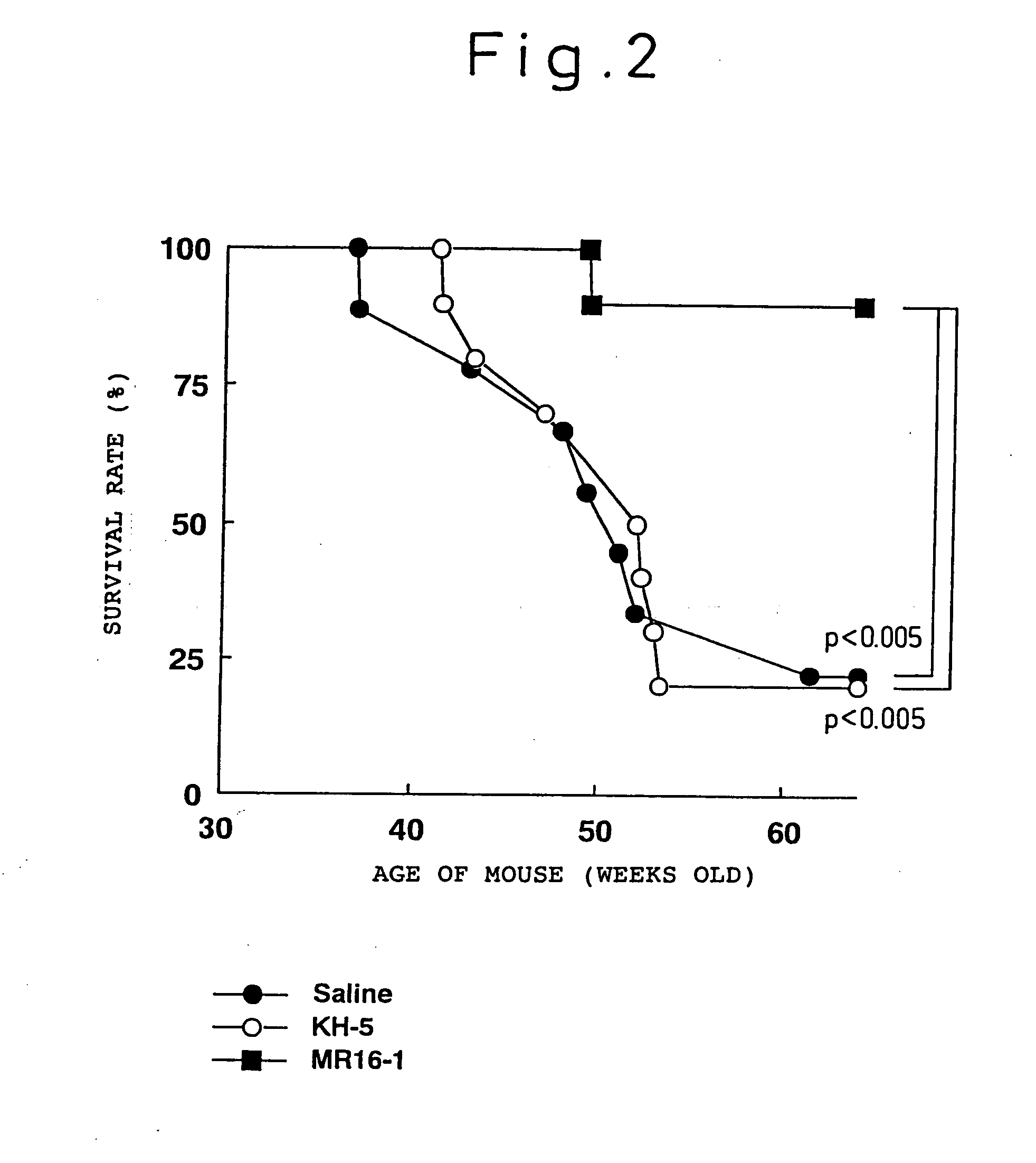
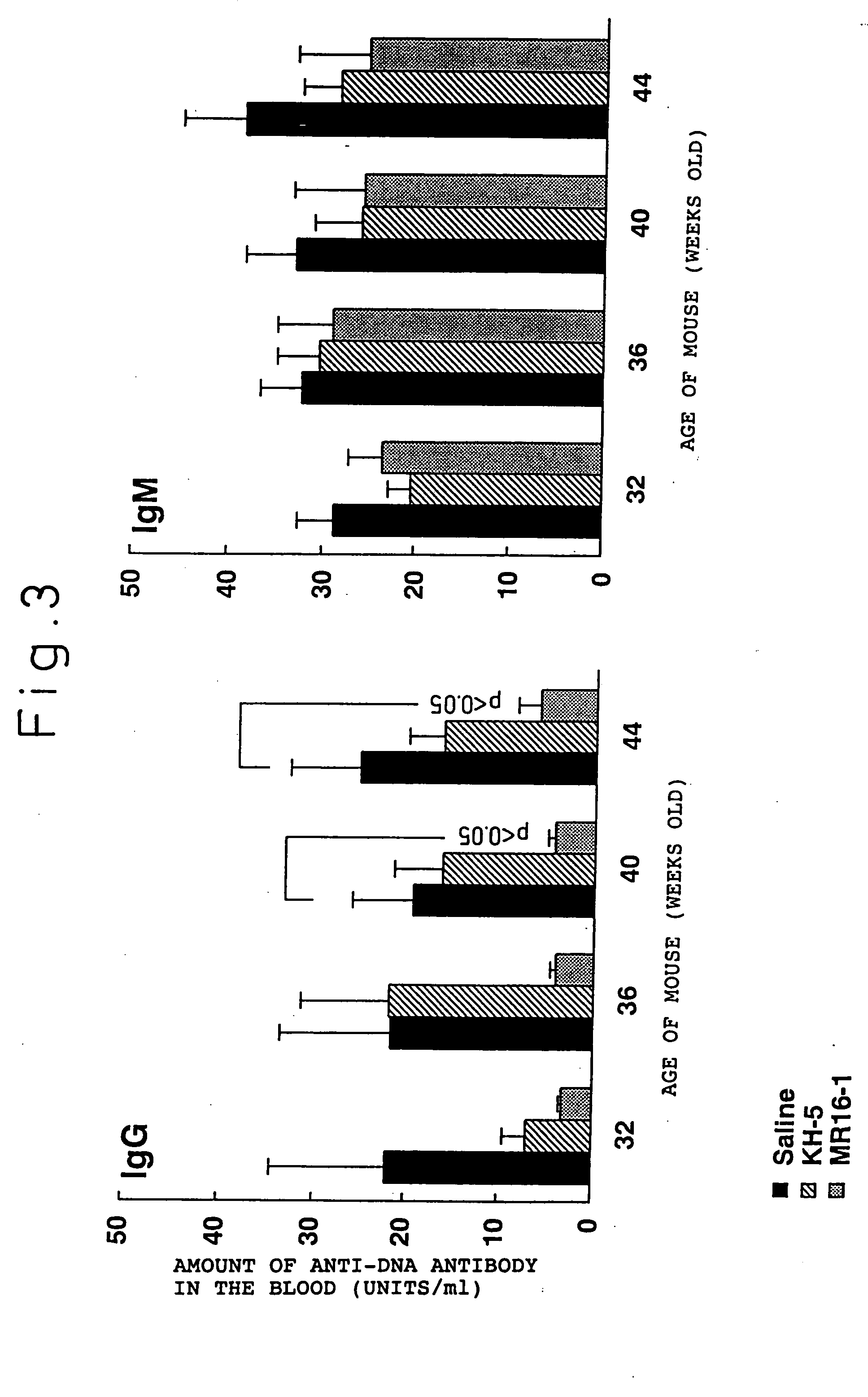
Preventive and/or therapeutic agent for systemic lupus erythematosus comprising anti-lL-6 receptor antibody as an active ingredient
a technology of anti-ll-6 receptor and active ingredient, which is applied in the direction of antibody medical ingredients, peptide/protein ingredients, immunological disorders, etc., can solve the problems of long-term administration, and achieve the effect of reducing anti-dna antibody or anti-nuclear antibody and reducing the excretion of urinary protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
Preparation of Human Soluble IL-6 Receptor
[0131] Soluble IL-6 receptor was prepared by the PCR method using a plasmid pBSF2R.236 containing CDNA that encodes IL-6 receptor obtained according to the method of Yamasaki et al., Science (1988) 241, 825-828. Plasmid pBSF2R.236 was digested with a restriction enzyme Sph I to obtain a cDNA of IL-6 receptor, which was then inserted into mp18 (manufactured by Amersham). Using a synthetic oligoprimer designed to introduce a stop codon into the cDNA of IL-6 receptor, a mutation was introduced into the cDNA of IL-6 receptor by the PCR method using an in vitro Mutagenesis System (manufactured by Amersham). The procedure resulted in the introduction of a stop codon to the amino acid at position 345, and gave a cDNA encoding soluble IL-6 receptor.
[0132] In order to express the cDNA of soluble IL-6 receptor in CHO cells, it was ligated to plasmid pSV (manufactured by Pharmacia) to obtain plasmid pSVL344. The cDNA of soluble IL-6 receptor that was...
reference example 2
Preparation of Human Anti-IL-6 Receptor Antibody
[0135] Anti-IL-6 antibody MT18 prepared by the method of Hirata et al. (J. Immunol., 143, 2900-2906, 1989) was bound to CNBr-activated Sepharose 4B (manufactured by Pharmacia Fine Chemicals, Piscataway, N.J.) according to the attached regimen and IL-6 receptor (Science (1988) 241, 825-8258) was purified. A human myeloma cell line U266 was solubilized with 1 mM p-para-aminophenyl methane sulfonyl fluoride hydrochloride (manufactured by Wako Chemicals) containing 1% digitonin (manufactured by Wako Chemicals), 10 mM triethanolamine (pH 7.8) and 0.15 M NaCl (manufactured by Wako Chemicals) (digitonin buffer), and mixed with MT18 antibody bound to Sepharose 4B beads. Then, the beads were washed six times with the digitonin buffer, to prepare the partially purified IL-6 receptor.
[0136] BALB / c mice were immunized four times every ten days with the above partially purified IL-6 receptor obtained from 3×109 U266 cells, and then a hybridoma wa...
reference example 3
Preparation of Mouse Anti-IL-6 Receptor Antibody
[0140] A monoclonal antibody directed against mouse IL-6 receptor was prepared according to the method described in Saito, et al., J. Immunol. (1993) 147,.168-173.
[0141] The CHO cells that produce mouse soluble IL-6 receptor were cultured in the IMDM culture liquid containing 10% FCS. From the culture supernatant, mouse soluble IL-6 receptor was purified using mouse soluble IL-6 receptor RS12 (see Saito, et al., supra) and an affinity column fixed to Affigel 10 gel.
[0142] The mouse soluble IL-6 receptor (50 μg) thus obtained was mixed with Freund's complete adjuvant, which was then injected to the abdomen of Wistar rats (Japan Charles River). From 2 weeks later the animals were boosted with Freund's incomplete adjuvant. On day 45, the rats were sacrificed, and about 2×108 spleen cells were fused with 1×107 mouse myeloma cells P3U1 using 50% PEG1500 (manufactured by Boehringer Mannheim) according to the conventional method, and then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com