Methods and processes for attaching compounds to matrices
a technology of compound and matrix, applied in the field of compound and matrix attachment, can solve the problems of long reaction time, substantial increase in cost, and rate-limiting step of initial attachment of chemical moiety to the matrix
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
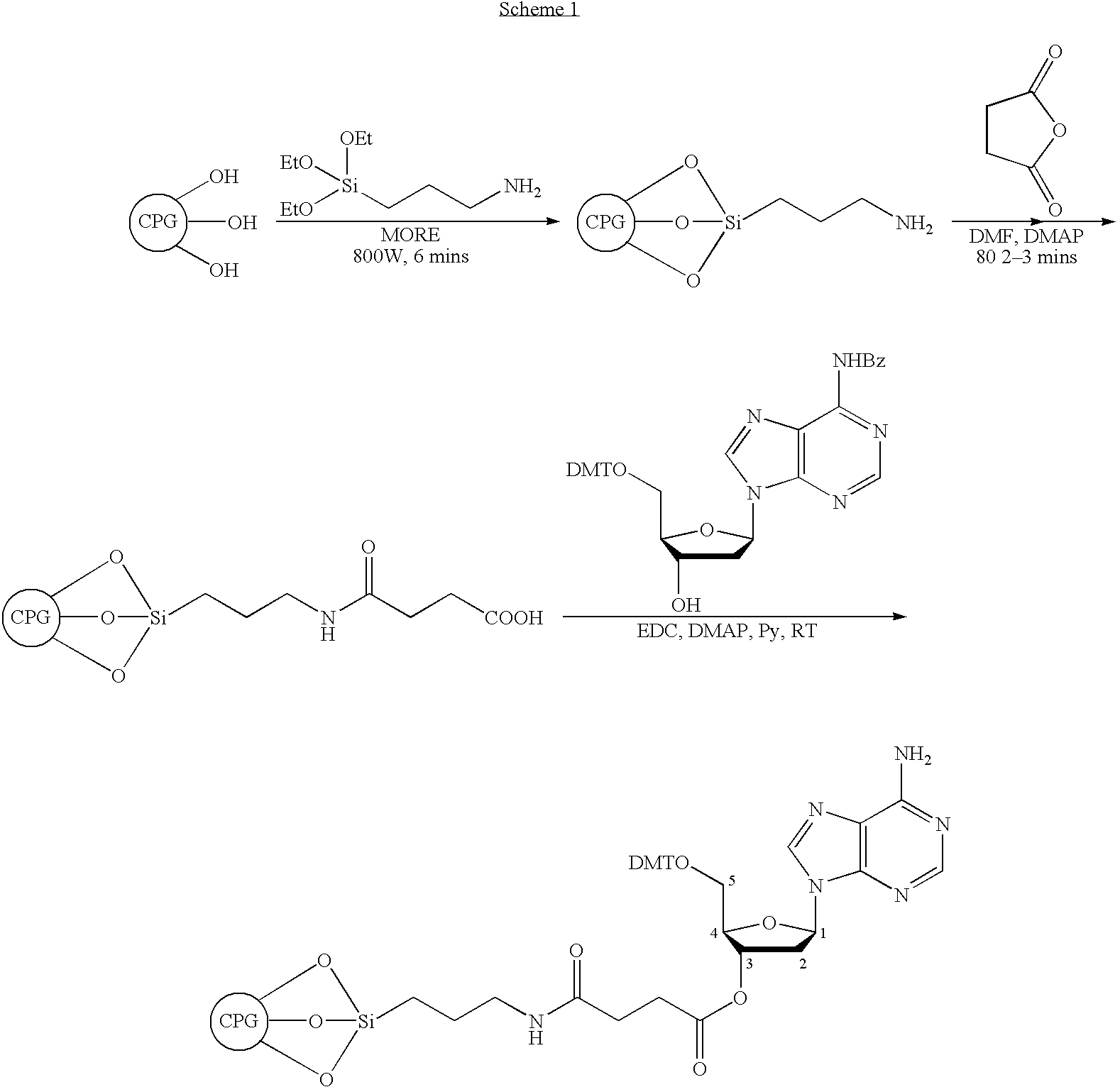
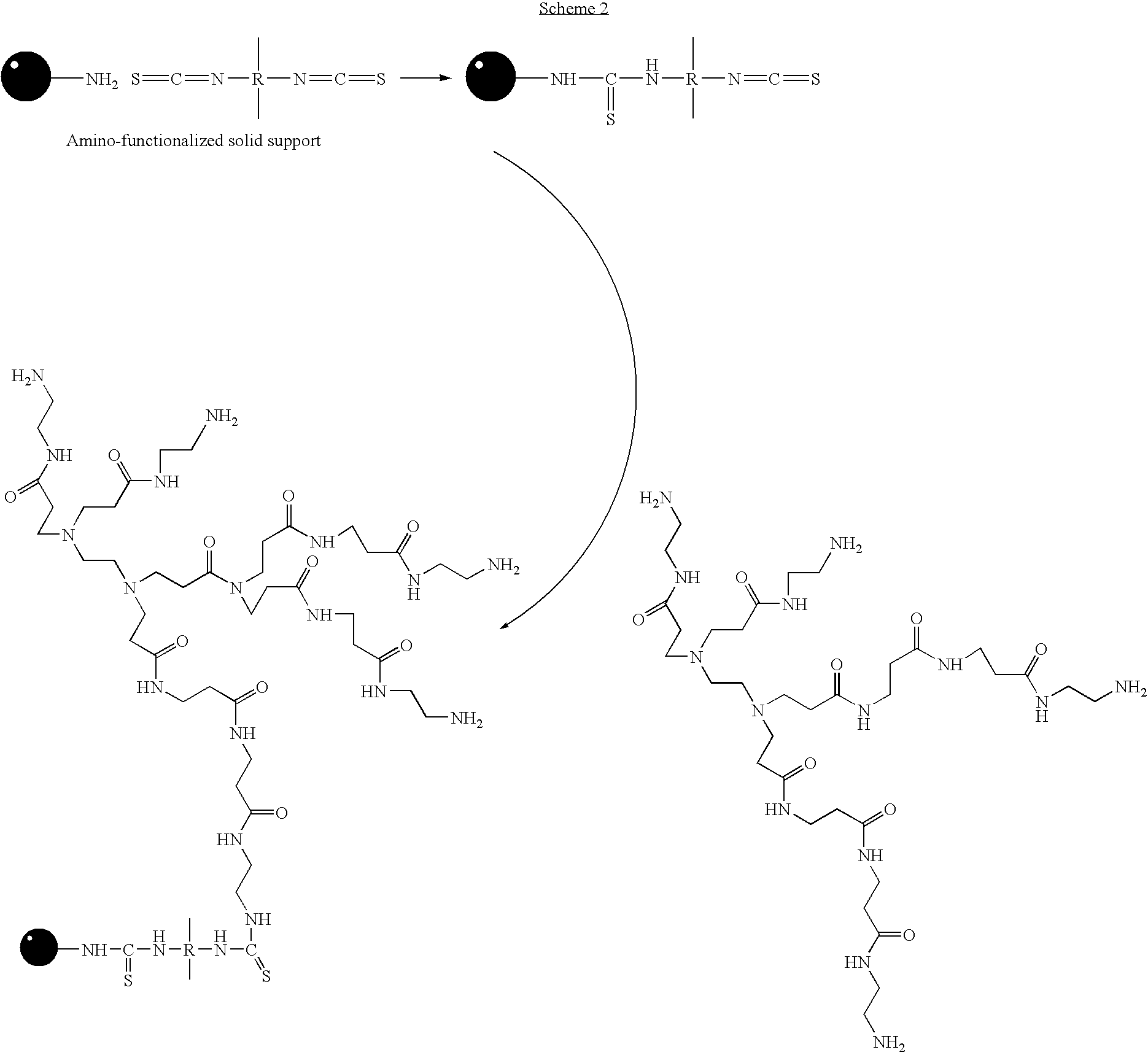
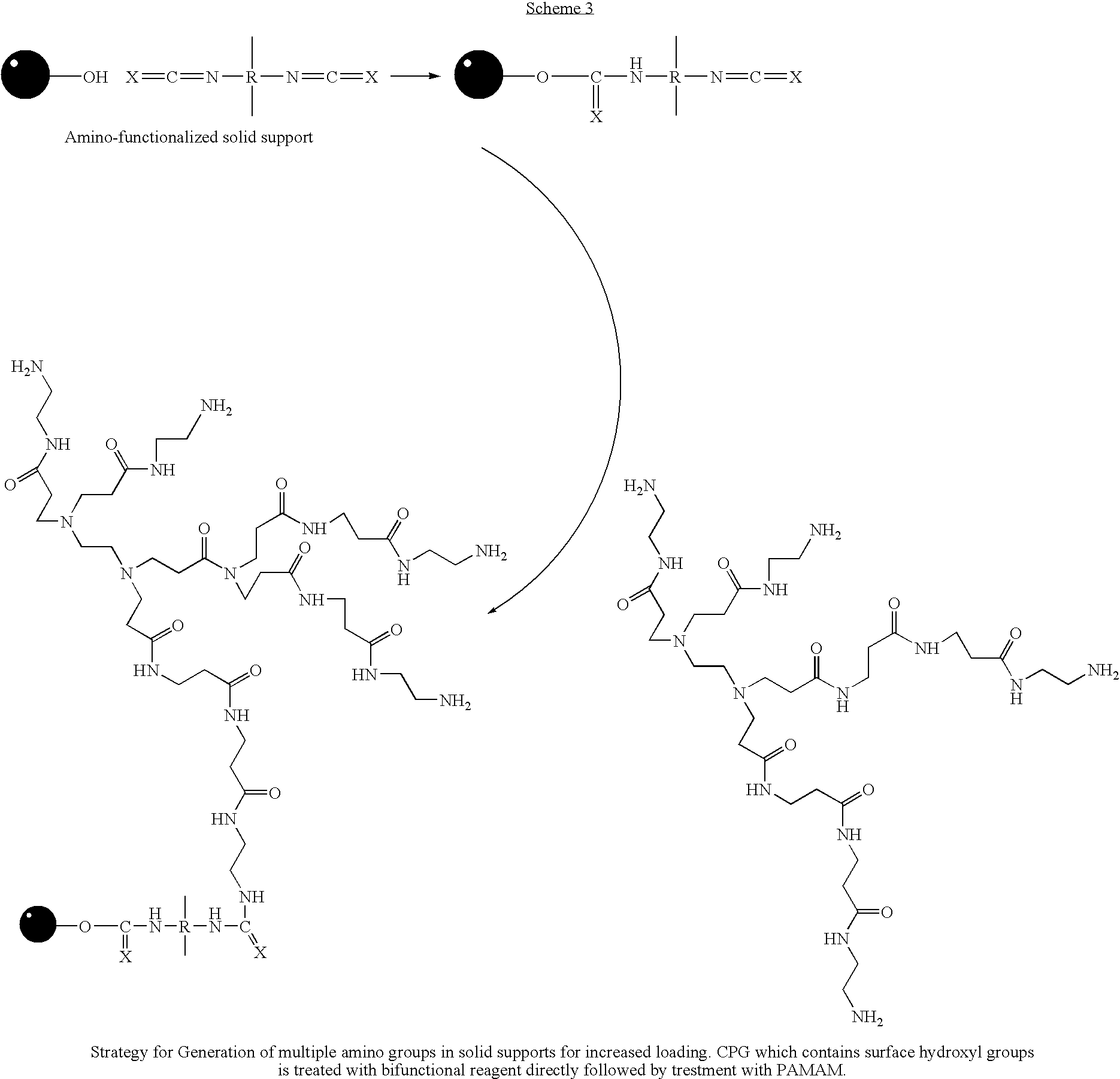
[0049] Rapid and efficient functionalization of solid-support by microwave-assisted procedures has been achieved. The functionalized solid-support can be readily loaded with nucleosides for use in oligonucleotide synthesis. This method can also be extended to rapid functionalization of other solid matrices for application in microarrays and combinatorial chemistry.
[0050] Over the past two decades, microwave-assisted procedures have been successfully employed in a number of synthetic transformations, resulting in rapid and efficient synthesis of different classes of organic compounds.1 Several advantages have been claimed in the use of microwave-assisted organic synthesis2: (a) ultra fast reaction kinetics, (b) cleaner reactions with improved yields and reduced formation of side products, (c) ability to effect, chemo-, regio-, and stereoselective transformations, (d) flexibility to perform reactions with or without solvents, (e) economical and eco-friendly processes than the corresp...
example 2
Amination of CPG:
[0095] In a pressure chamber equipped with a Teflon plug and a chemically resistant 0 ring (Chemraz), 3-aminopropyltriethoxysilane (APTES, 450 ml, ‘3.5 ml / g) was added to Native CPG 500 CPG (1 35g) and this well mixed reaction mixture was heated in a house-hold microwave (800 watt) in 1 min cycle for 8 mins. The contents of reaction mixture was mixed well by shaking between the heating cycles and allowed to cool intermittently. At the end of heating, the reaction mixture was allowed to cool to RT, filtered, washed with toluene (2×125 ml) followed by methanol (2×250 ml), dichloromethane (2×250 ml) and finally with hexanes (2×250 ml). Washed sample was treated with drops of ninhydrin and heated and the presence of amino group was indicated by strong purple color. The aminated CPG was dried in a glass tray and a small sample was dried under high vacuum overnight for amine loading by reported procedure through dimethoxytrityl content analysis as follows. Trityl analys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| dielectric constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com