Immunotoxins directed against malignant cells
a technology of immunotoxins and malignant cells, applied in the direction of antibody medical ingredients, peptide/protein ingredients, peptide sources, etc., can solve the problems of stymied utilization of this type of anti-tumor therapy, undesired side effects, and traditional chemotherapies, and achieve dramatic lowering of side effects and surprising properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Native and Recombinant Onc Protein from Rana pipiens
[0093] A. Isolation and Purification of Native Onc Protein
[0094] Techniques describing the isolation of oocytes from Rana pipiens, in vitro fertilization of the eggs, and the isolation and purification of native onc protein from frog embryos are exquisitely detailed in U.S. Pat. Nos. 5,559,212 and 5,728,805, which are both incorporated by reference herein.
[0095] B. Production and Assaying of Recombinant Onc Protein
[0096] The production of recombinant onc protein was done as described in PCT application PCT / US97 / 02588. Ribonucleolytic activity using high molecular weight RNA and tRNA was determined following published protocols, Newton, et al., J. Neurosci. 14:538 (1994) at 37° C. through the formation of perchloric acid soluble nucleotides (see, Newton, et al., Biochem. 35:545 (1996)). With poly (A,C), UpG, and poly U, ribonuclease activity was assayed spectrophotometrically according to Libonati, et al., Biochim....
example 2
Chemical Analysis and Composition of Onc Proteins
[0097] The native onc protein described above has been well characterized chemically. To be as fully functional as the native onc protein, it is believed the recombinant onc protein should have the chemistry and structure as described below.
[0098] The native onc protein was purified to homogeneity (as established by standard tests used to assay the homogeneity of proteins). By electrophoresis, the molecular weight of the native onc protein was determined to be approximately 14,500 Daltons. Calculation of the molecular weight based upon the listed amino acid sequence (see, infra), indicated the molecular weight of native onc protein should be 11,860 Daltons. However, because metal ions may have bonded to the protein despite all efforts to remove them, and because different isotopes may be involved, the molecular weight of the native onc protein was 12,430 Daltons as determined by mass spectroscopy. In view of this discrepancy, the mo...
example 3
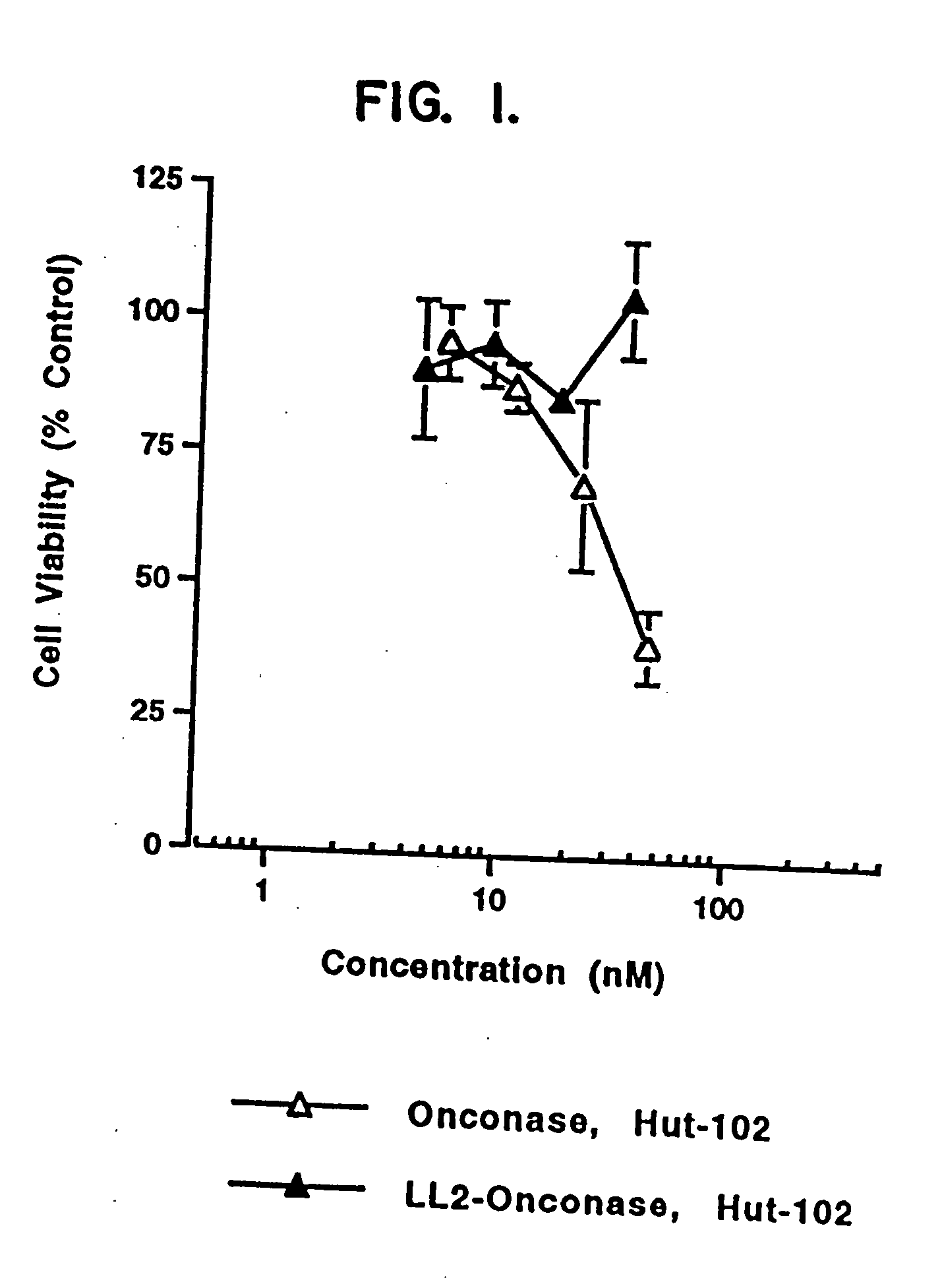
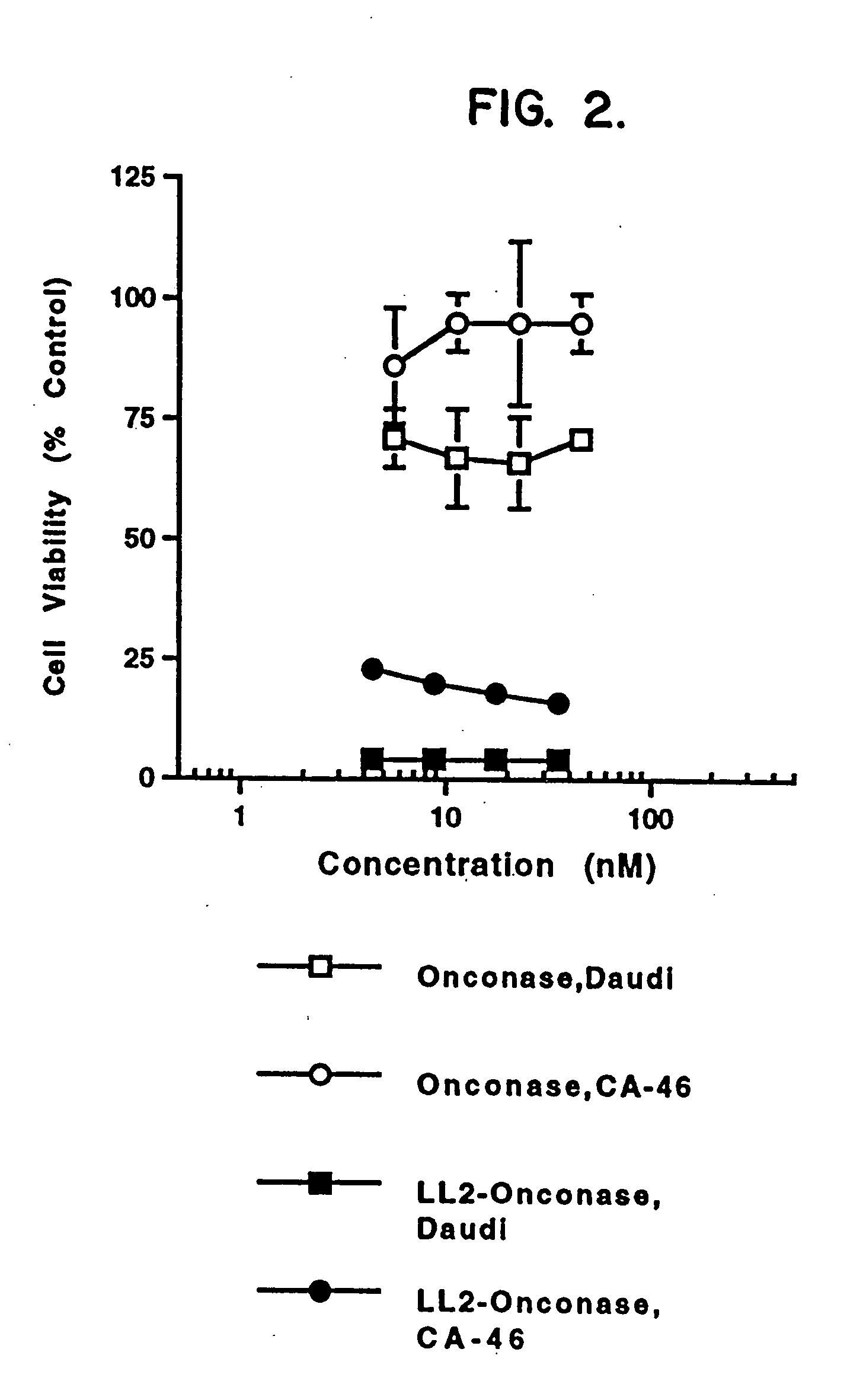
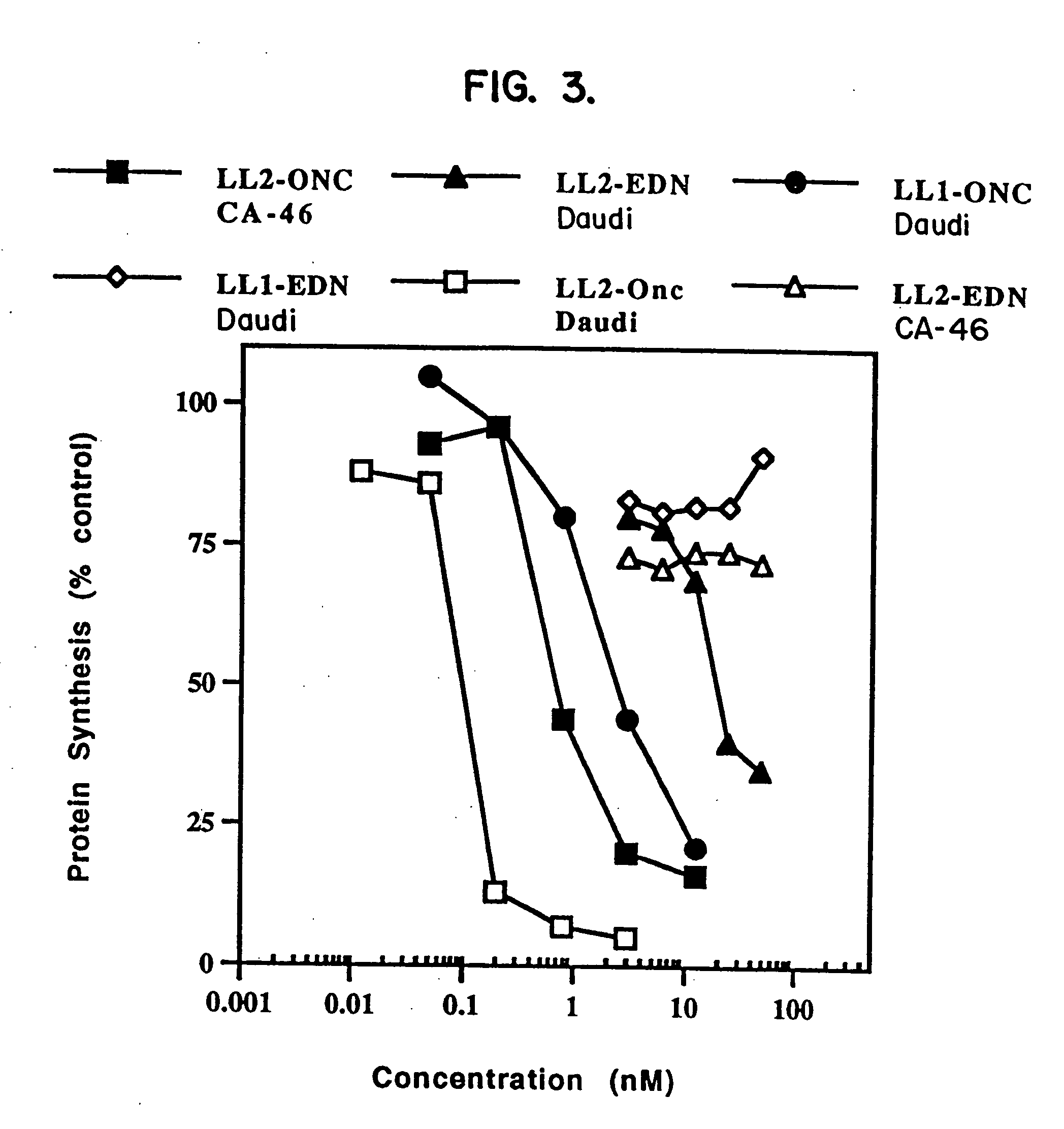
ANTI-CD22-ONCONASE® Immunotoxin
[0102] A. Materials and Methods
[0103] ONCONASE® (previously named P-30) was provided by Alfacell Corp. as a lyophilized protein and was dissolved in phosphate buffered saline (PBS). Stock solutions of at least 1 mg / mL were kept frozen at −20° C. until dilutions were prepared for assays. All other reagents were purchased from sources previously described (Rybak, et al., J. Biol. Chem. 266:21202 (1991); Newton, et al., J. Biol. Chem. 267:19572 (1992); Mikulski, et al., Cell Tissue Kinet. 23:237 (1990)), herein incorporated by reference.
[0104] LL2 is a murine monoclonal antibody that recognizes and specifically binds to CD22 on human B cells. The LL2 antibody was provided by Immunomedics, Inc. (Morris Plains, N.J.). RFB4 is also a murine monoclonal antibody that binds to CD22. This antibody is available from many sources, including Ancell Corp.
[0105] Three Burkitt lymphoma cell lines (Daudi (ATCC CCL 213), CA 46 (ATCC CRL 1648), and Raji (ATCC CCL86))...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com