Methods and cells for expression of recombinant protein products under the transcriptional control of an inducible promoter
a technology of inducible promoter and recombinant protein, which is applied in the field of methods and cells for expression of recombinant protein products under the transcriptional control of inducible promoter, can solve the problems of low protein expression level of uninduced ara system, inability to modulate the expression of cloned genes, and inability to induce ara system protein expression, etc., to achieve efficient and economical production, increase the expression of recombinant protein products, and reduce the effect of ara
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Arabinose Transport-Deficient Strains
[0039] Isogenic ΔaraFGH::kan and araE201 strains were constructed from E. coli W3110 (ATCC 27325) and E. coli E104 (deposited as ATCC 69009; ATCC 69008; ATCC 69101; ATCC 69102; ATCC 69103; ATCC 69104; ATCC 69331; ATCC 69332; ATCC 69333, each of which contains a gelonin-encoding plasmid). The resultant strains along with intermediate strains are shown in Table 1.
TABLE 1Arabinose Proficient and Deficient StrainsStrainParent or ReferenceRelevant GenotypeCW2553Horazdovsky and Hogg, 1989,ΔaraFGH::kan araE201Jour. of Bacter. 171: 3053-3059E104W3110 / See, Example 1Δ(araBC)768W3110ATCC 27325E220W3110 / PTA-1524AΔaraFGH::kanE221E104 / PTA-1525AΔaraFGH::kanE222W3310thyAE223E104thyAE224E222 / PTA-1526AaraE201E225E223 / PTA-1527AaraE201E226E224 / E228 / PTA-1528AΔaraFGH::kan araE201E227E229 / PTA-1529AΔaraFGH::kan araE201E228E220ΔaraFGH::kan thyAE229E221ΔaraFGH::kan thyA
ADeposited with the American Type Culture Collection (ATCC) on Mar. 21, 2000.
Constr...
example 2
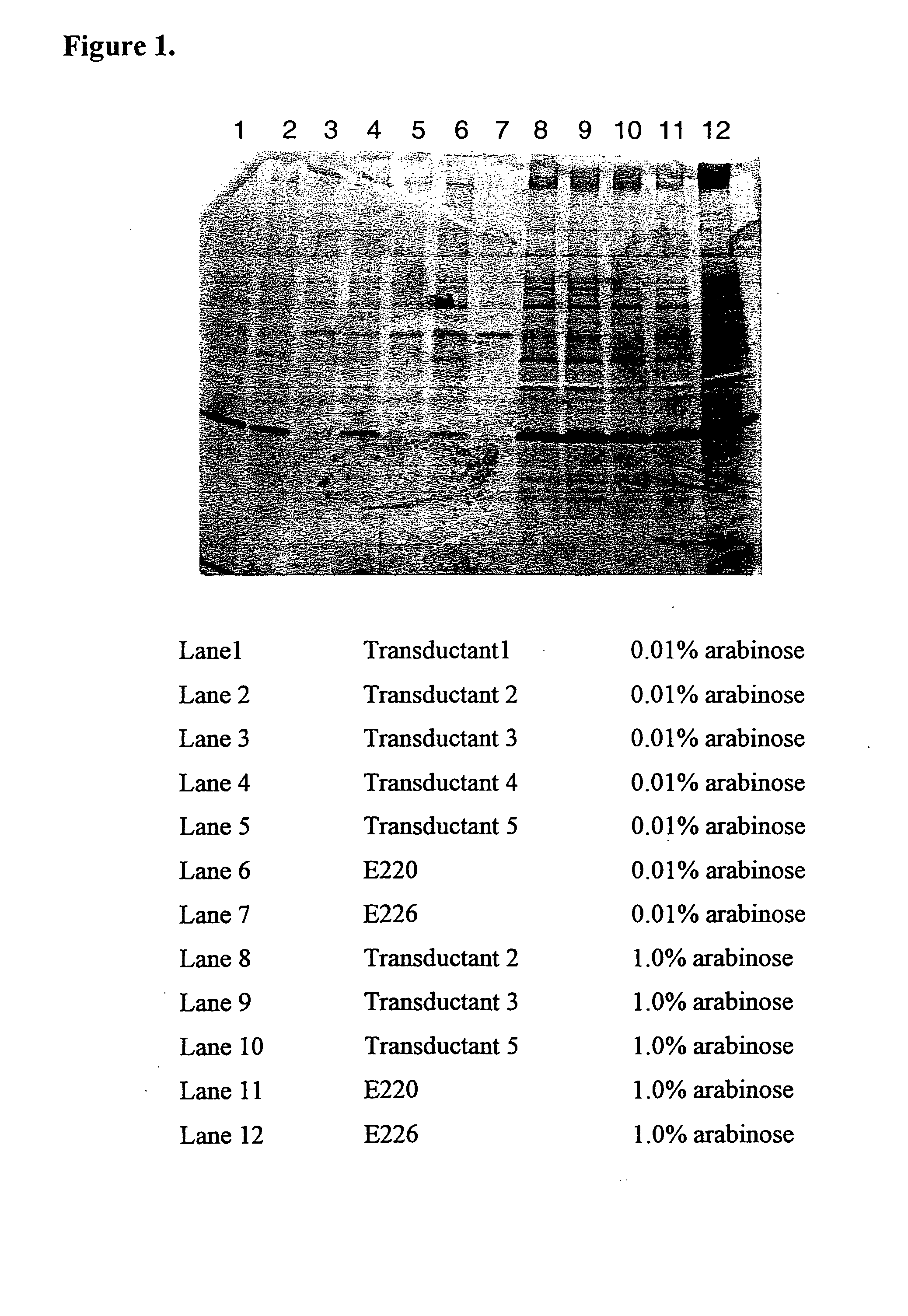
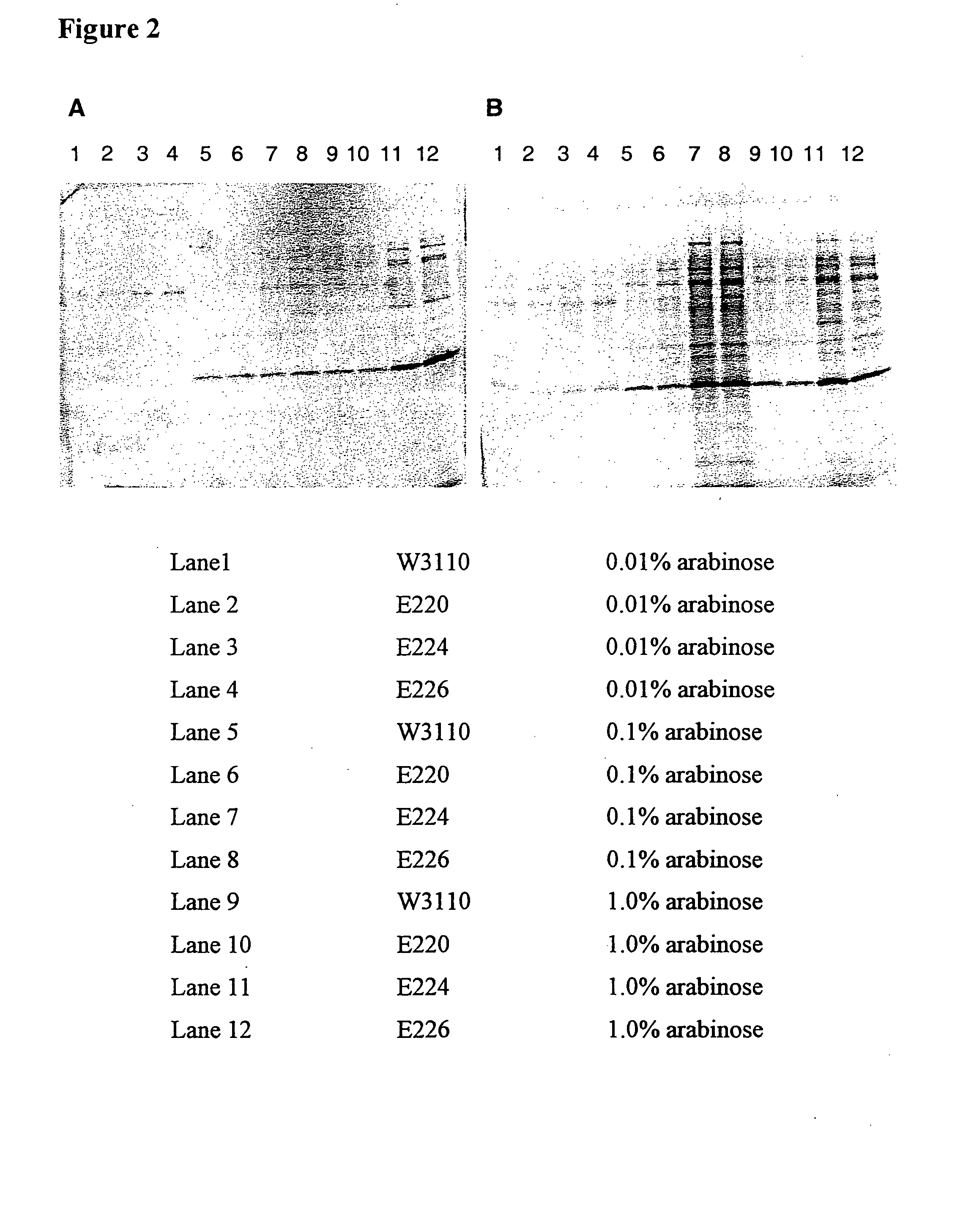
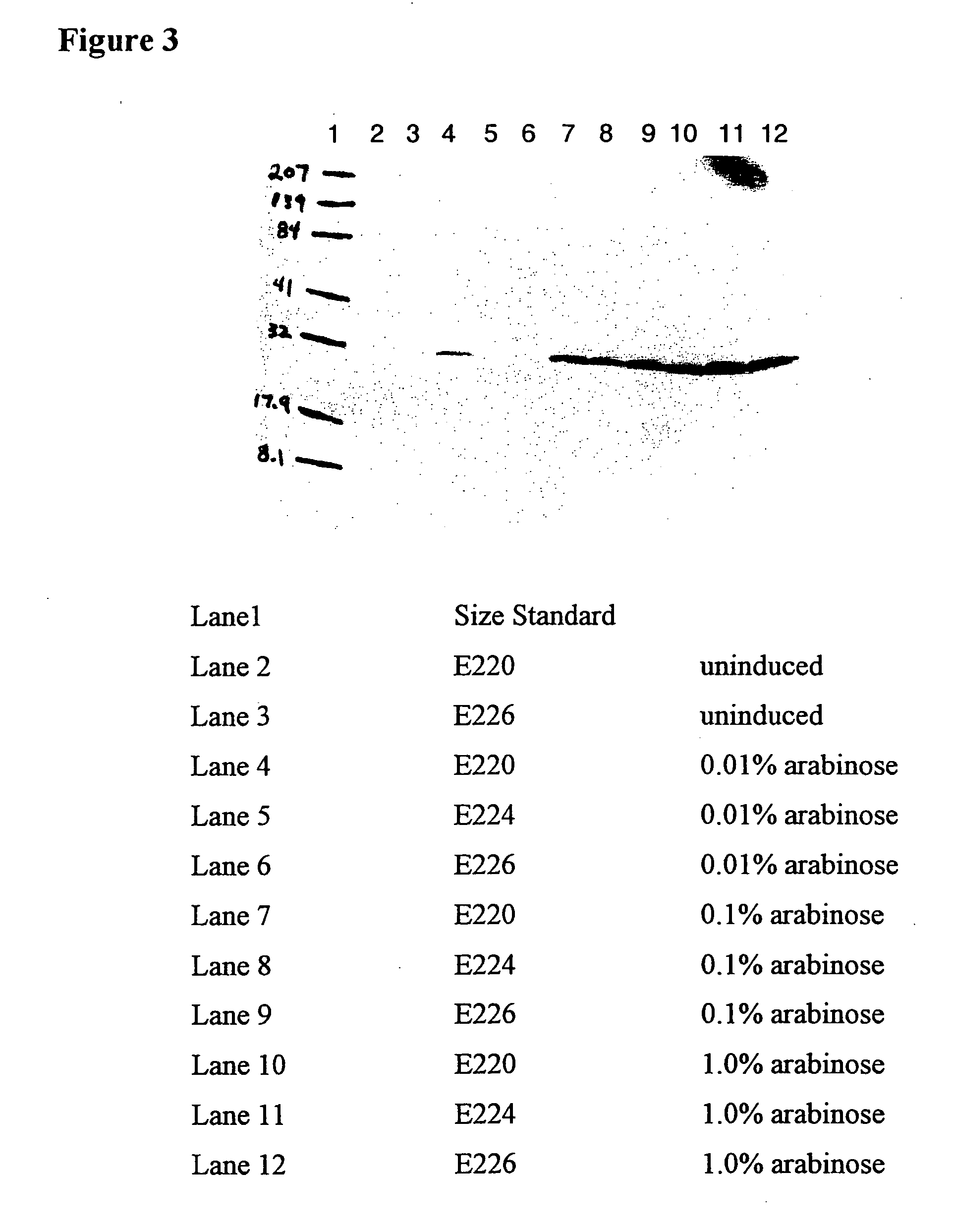
SDS-PAGE and ELISA Analysis of Gelonin Expression in W3110, E220, E224 and E226 after Arabinose Induction
[0047] Four isogenic strain (W3110, E220, E224 and E226) were transformed with pING3825. Each cell line was grown to an OD600 of approximately 0.4, then aliquots of 2 mL of each culture were transferred to tubes supplemented with arabinose to 0, 0.01, 0.1 or 1%. Twenty eight tubes were set up, so that cells induced with 0, 0.01, 0.1 or 1% arabinose could be harvested at 4 hours post-induction and cells induced with 0.01, 0.1 and 1% arabinose could be harvested 18 hours post-induction. After the appropriate induction period of either 4 or 18 hours, cells were removed from the culture supernatant by centrifugation and each supernatant was filtered through a 0.2 μm filter before storage at 4° C. Ten microliters of each supernatant was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Samples from each supernatant were stained with Coomassie colloida...
example 3
Analysis of Cell Growth and Gelonin Expression in E104, E221, E225 and E227 after Arabinose Induction
[0052] Four Δ(araBD)768 bacterial cell lines (E104, E221, E225 and E227) each containing pING3825 were grown in TYE medium to an OD of approximately 0.4 and induced in 2.15 mL aliquots with arabinose to 0, 0.1, 1 and 10 mM (10 mM 0.15%; 1 mM 0.015%; 0.1 mM 0.0015%). At 4 hours post-induction, the OD600 of a 1:5 dilution of each culture was determined, then the culture was centrifuged to remove cells and the filtered supernatant was placed at −20° C. for subsequent evaluation by ELISA. Likewise, at 17 hours post-induction, the OD600 of a 1:10 dilution of each culture was determined, then the culture was centrifuged to remove cells and the filtered supernatant was placed at −20 C for subsequent evaluation by ELISA. Table 4 illustrates the culture OD600 for each sample.
TABLE 4Optical Density of E104, E221, E225 and E227 cultures inducedwith arabinoseStrainmM arabinoseOD (4 hours)OD (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cell density | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com