Process for low temperature, dry etching, and dry planarization of copper
a technology of copper and dry etching, applied in the direction of decorative arts, decorative surface effects, electrical equipment, etc., to achieve the effect of rapid, cost-effective and environmental-friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
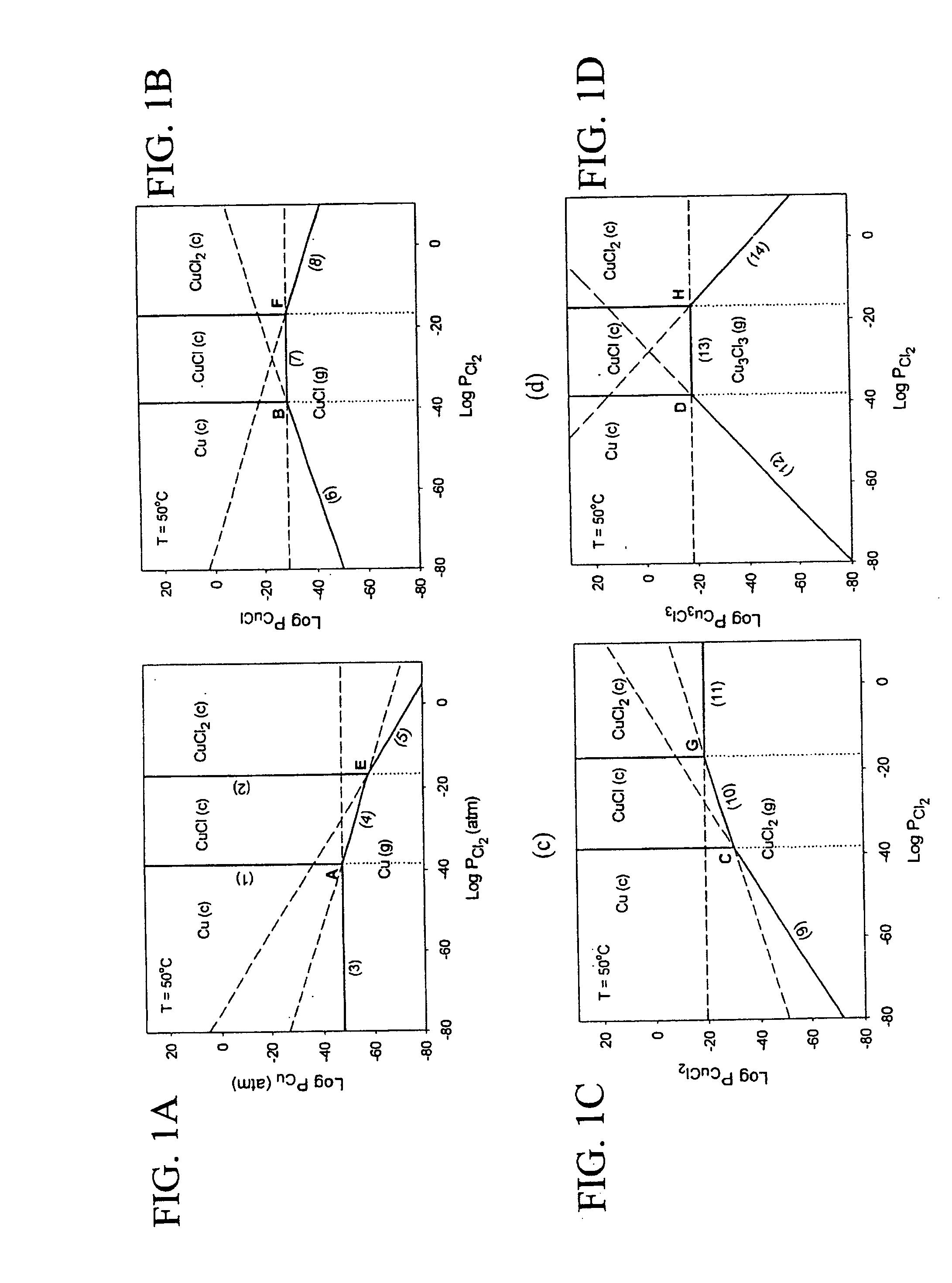
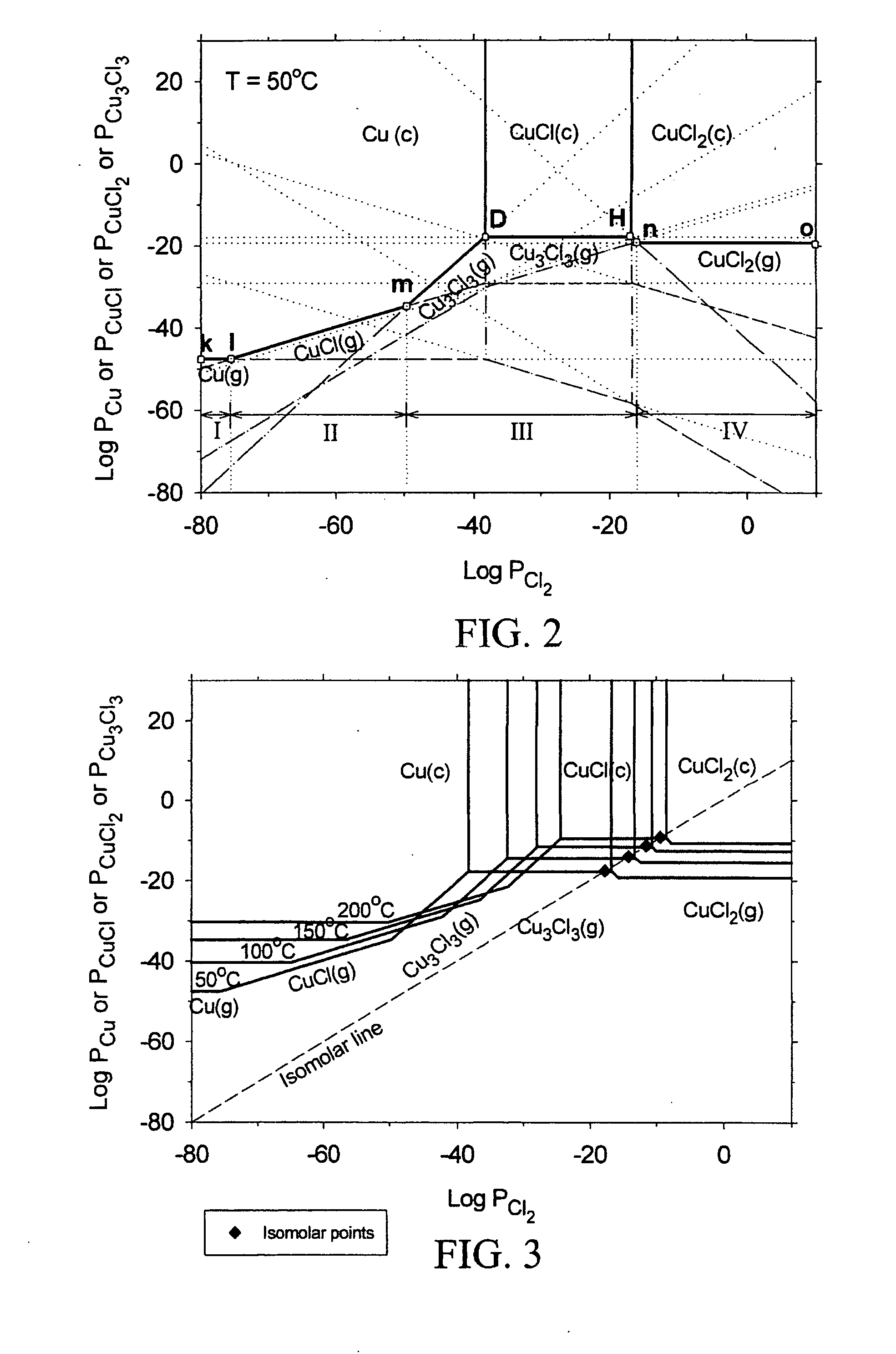
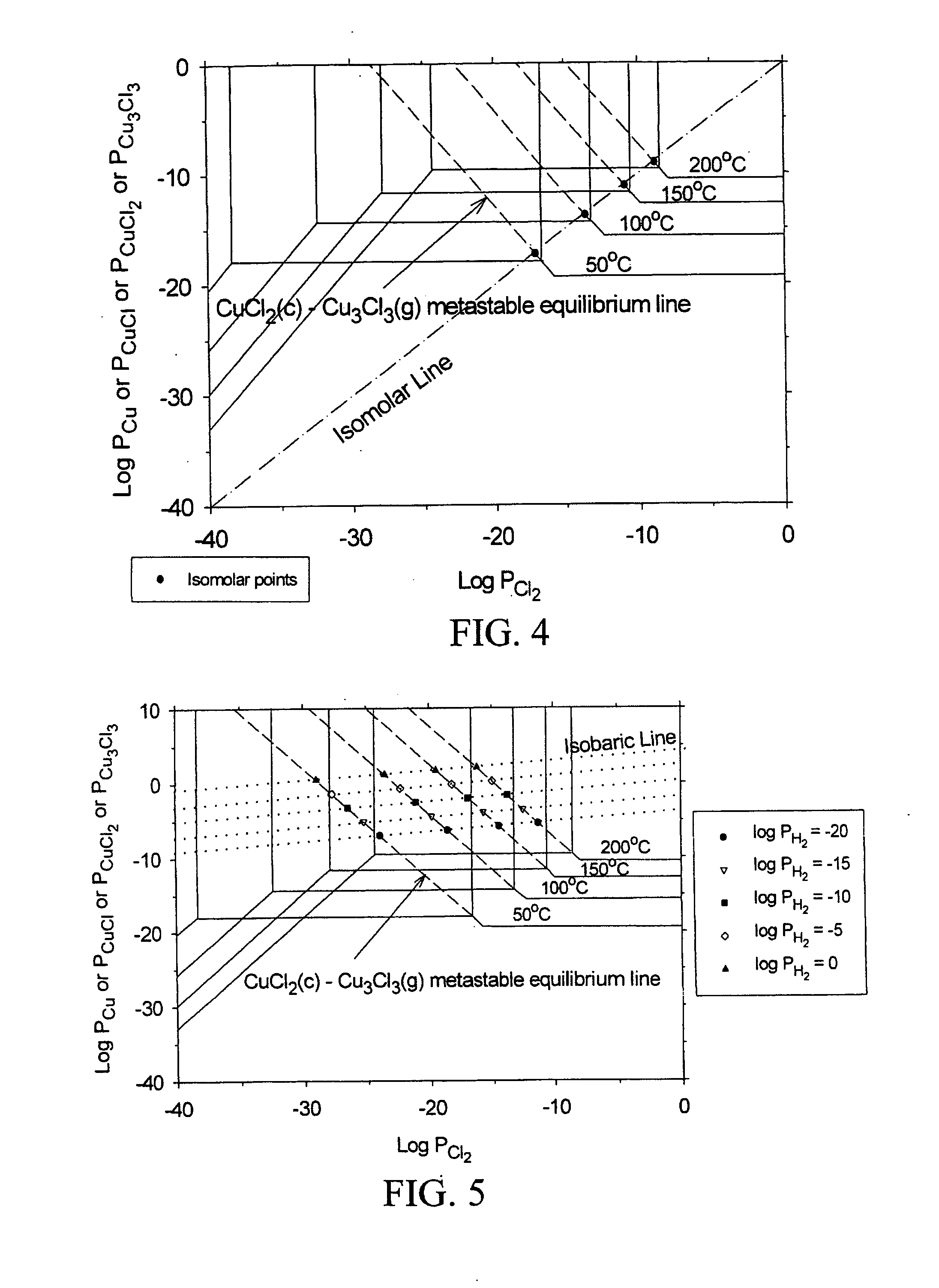
[0024] The subject invention pertains to a method and apparatus for etching copper (Cu). The subject invention can involve passing a halide gas over an area of Cu such that CuX, or CuX and CuX2, are formed, where X is the halide. Examples of halide which can be utilized with the subject matter include, but are not necessarily limited to, Cl, Br, F, and I. Once the CuX, or CuX and CuX2, are formed the subject invention can then involve passing a reducing gas over the area of Cu for a sufficient time to etch away at least a portion of the CuX, or CuX2 are produced when the halide gas is passed over the area of Cu, the reducing gas can be passed until essentially all of the CUX2 is etched and at least a portion of the CuX is etched. Examples of reducing gases which can be utilized with the subject invention include, but are not necessarily limited to, hydrogen gas and hydrogen gas plasma. The subject invention can accomplish the etching of Cu by passing the reducing gas over the Cu so ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com