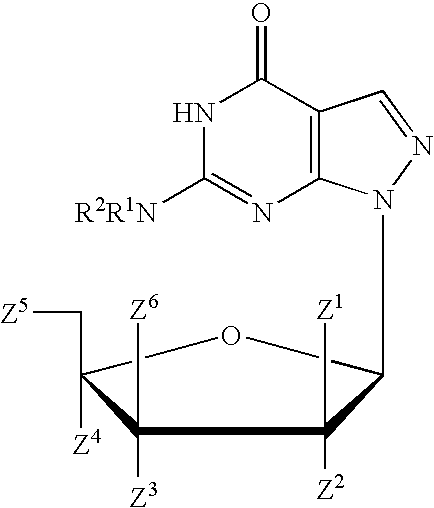
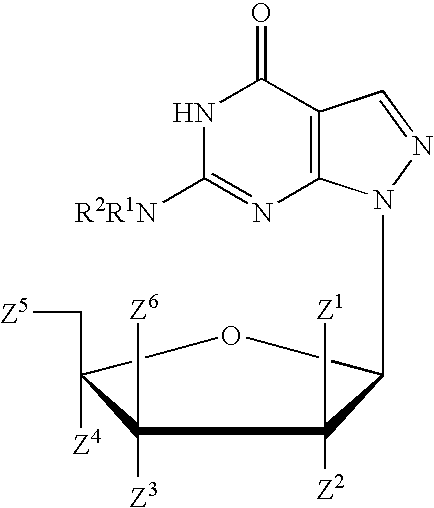
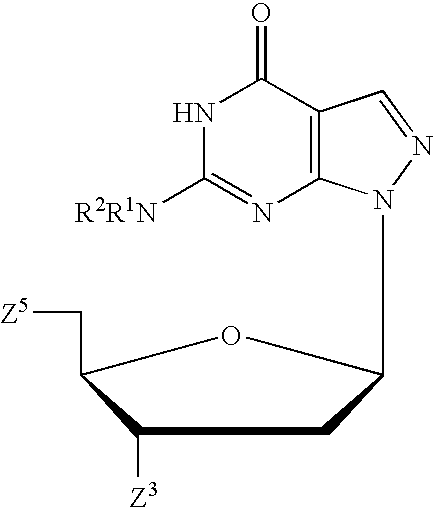
Process for the synthesis of pyrazolopyrimidines
a technology of pyrazolopyrimidine and pyrazolopyrimidine, which is applied in the field of pyrazolopyrimidine production, can solve the problems of increasing the production cost of ppg phosphoramidite, chromatography purification of a compound is not suitable for a large volume, and the chromatography process is generally time-consuming and laborious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0077]
[0078] This example illustrate a method for producing 2-amino-4,6-dichloropyrimidine-5-carboxaldehyde (2).
[0079] Absolute DMF (210 ml, 1.38 mol) was added drop wise to an ice-cold solution of POCl3 (900 ml) over a 20 min period. The ice-bath was removed and 150 g (1.17 mol) of powdered 2-amino-6-hydroxypyrimidine-4(3H)-one (1) was added over a 20 min period. The mixture was then heated to 100° C. and stirred for 3-4 h. The solution was cooled to room temperature and poured into 10 L of ice water (4 L of crushed ice diluted to 10 L with water). This aqueous solution was then heated to 50° C. and stirred for 2 h. The mixture was refrigerated overnight and the precipitate was filtered and dried to provide 160 g (71% yield) of 2-amino-4,6-dichloropyrimidine-5-carboxaldehyde (2).
example 2
[0080]
[0081] This example illustrate a method for producing 4-chloro-6-aminopyrazolo[3,4-d]pyrimidine (3).
[0082] To a mixture of 2-amino-4,6-dichloropyrimidine-5-carboxaldehyde (2) (150 g, 0.785 mol), THF (2.7 L) and triethylamine (125 ml) was added anhydrous hydrazine (25.5 ml in 600 ml of water) drop wise over 25 min. Alternatively, hydrazine monohydrate can be used instead of anhydrous hydrazine. The mixture was stirred for 1 h. The solids were filtered off and the filtrate was evaporated to remove about 80% of the THF. Water was added and the resulting precipitate was filtered and dried under vacuum. The solid was then dissolved in a minimum volume of hot DMF and precipitate by addition of water to provide 112 g (85% yield) of 4-chloro-6-aminopyrazolo[3,4-d]pyrimidine (3).
example 3
[0083]
[0084] This example illustrates a method for producing 4-methoxy-6-aminopyrazolo[3,4-d]pyrimidine (4).
[0085] Compound 3 (70.4 g, 0.416 mol) was refluxed in methanolic sodium methoxide solution (25 g sodium dissolved in 1 L methanol) for 2 h. The reaction mixture was cooled to room temperature and neutralized by addition of acetic acid (75 ml). The mixture was evaporated to dryness and the solid was triturated in 600 ml of water, filtered and dried to provide 67.5 g (99% yield) of 4-methoxy-6-aminopyrazolo[3,4-d]pyrimidine (4).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com