Production of chimeric bovine or porcine animals using cultured inner cell mass
a technology of inner cell mass and which is applied in the field of production of chimeric bovine or porcine animals using cultured inner cell mass, can solve the problems of insufficient cell culture, inability to produce transgenic ungulate embryos produced from transgenic es cells, and inability to manipulate or otherwise control dna integration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
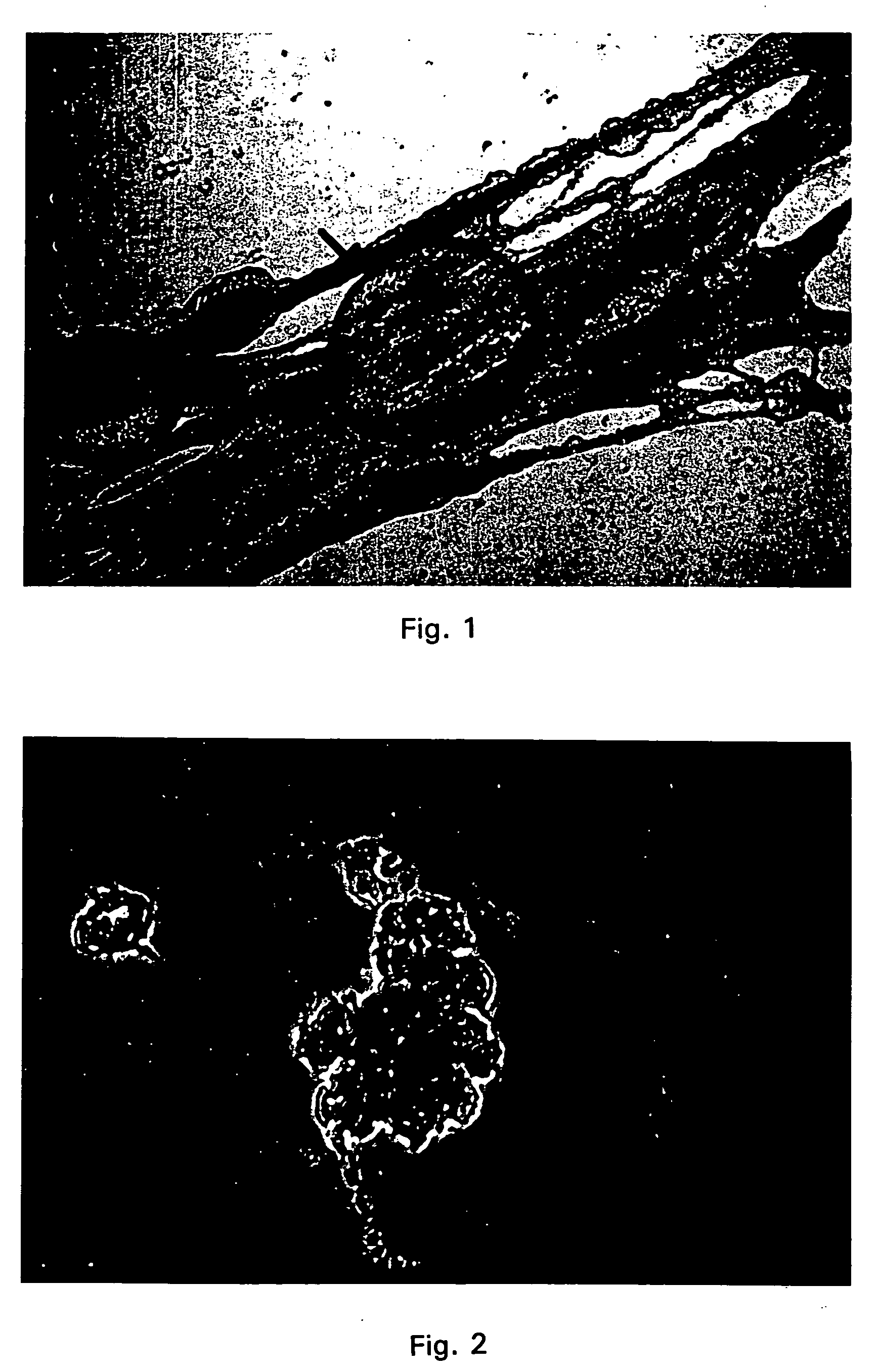
[0177] Production of CICM cell lines from pre-blastocyst and blastocyst stage pig embryos were conducted using the following general protocol. First, primary cultures of embryonic fibroblasts were obtained from 12-16 day old murine fetuses. After the head, liver, heart and alimentary tract were aseptically removed, the embryos were minced and incubated for 30 minutes at 37° C. in prewarmed trypsin EDTA solution (p. 05% trypsin / 0.02% EDTA; GIBCO, Grand Island, N.Y.). Fibroblast cells were plated in tissue culture dishes and cultured in alpha-MEM medium (BioWhittaker, Walkersville, Md.) supplemented with 10% fetal calf serum (FCS) (Hyclone, Logen, Utah) penicillin (100 IU / ml) and streptomycin (50 μg / ml). Three to four days after passage, embryonic fibroblasts, in 35×10 Nunc culture dishes (Baxter Scientific, McGaw Park, Ill.), were treated with mitomycin C (10 μg / ml; Sigma) in supplemented alpha MEM for a minimum of three hrs. The fibroblasts were grown and maintained in a humidified ...
example 2
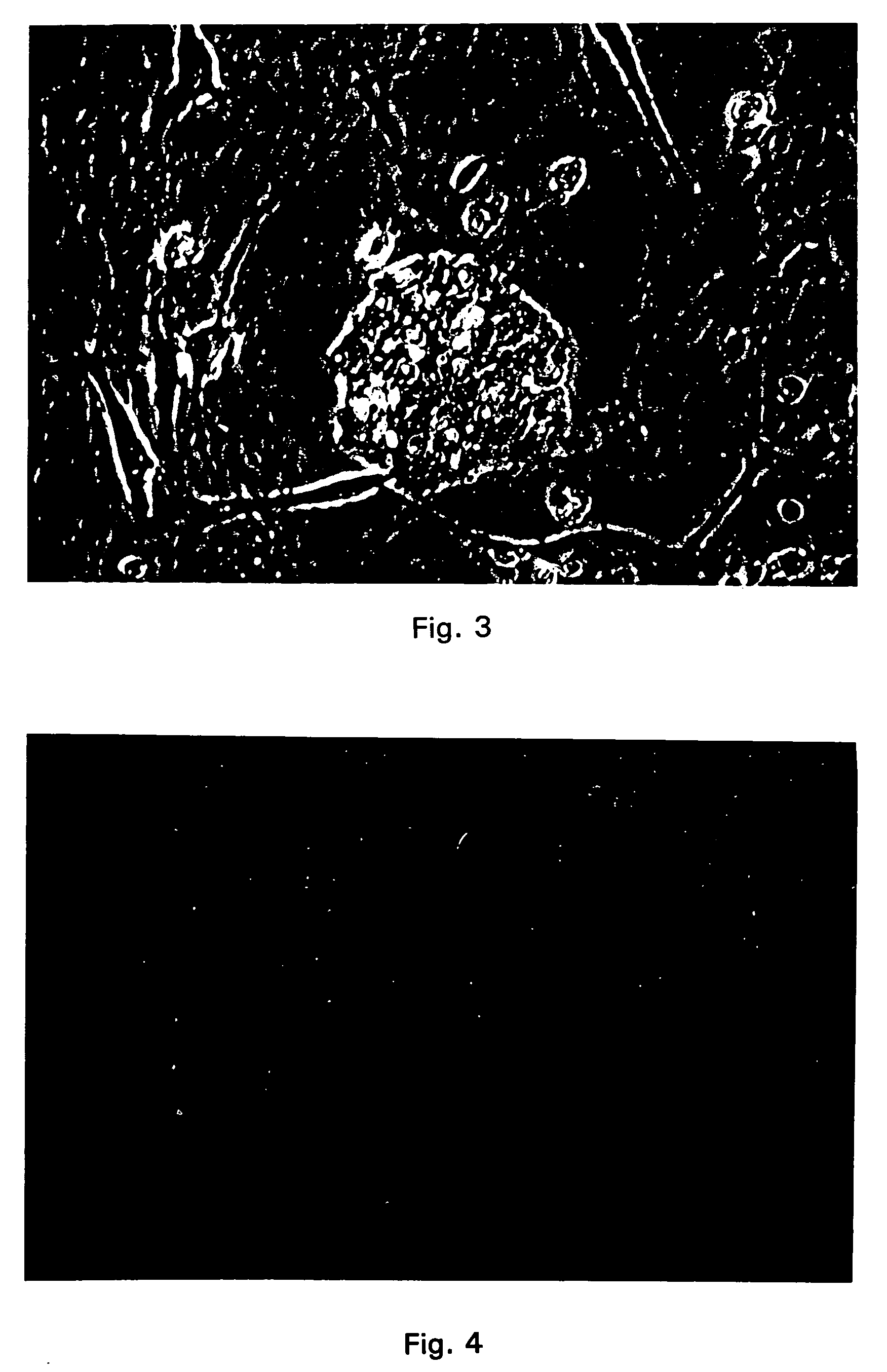
[0184] CICM cells obtained according to Example 1 were used for insertion of heterologous DNA's. Specifically, these cells were microinjected with linear as well as supercoiled DNA constructs containing different promoters placed in front of either the beta-galactosidase gene and / or the neomycin phosphotransferase gene. The specific promoters used were the cytomegalovirus promoter (CMV promoter), phosphoglycerate Kinase promoter (PGK promoter), mammary promoter (MAM promoter), reCMV promoter and chicken beta actin promoter. These gene constructs were diluted in a buffered solution (containing 80 mM KCl and 70 mM HEPES.) However, other buffers may readily be substituted therefore, such as Tris EDTA. The concentration of the DNA constructs in solution ranged from 5 to 10 μg / ml. However, concentrations ranging from 0.1 to 100 μg / ml should be effective. These DNA preparations were then microinjected into cultured CICM cells obtained according to Example 1.
[0185] In this procedure, the ...
example 3
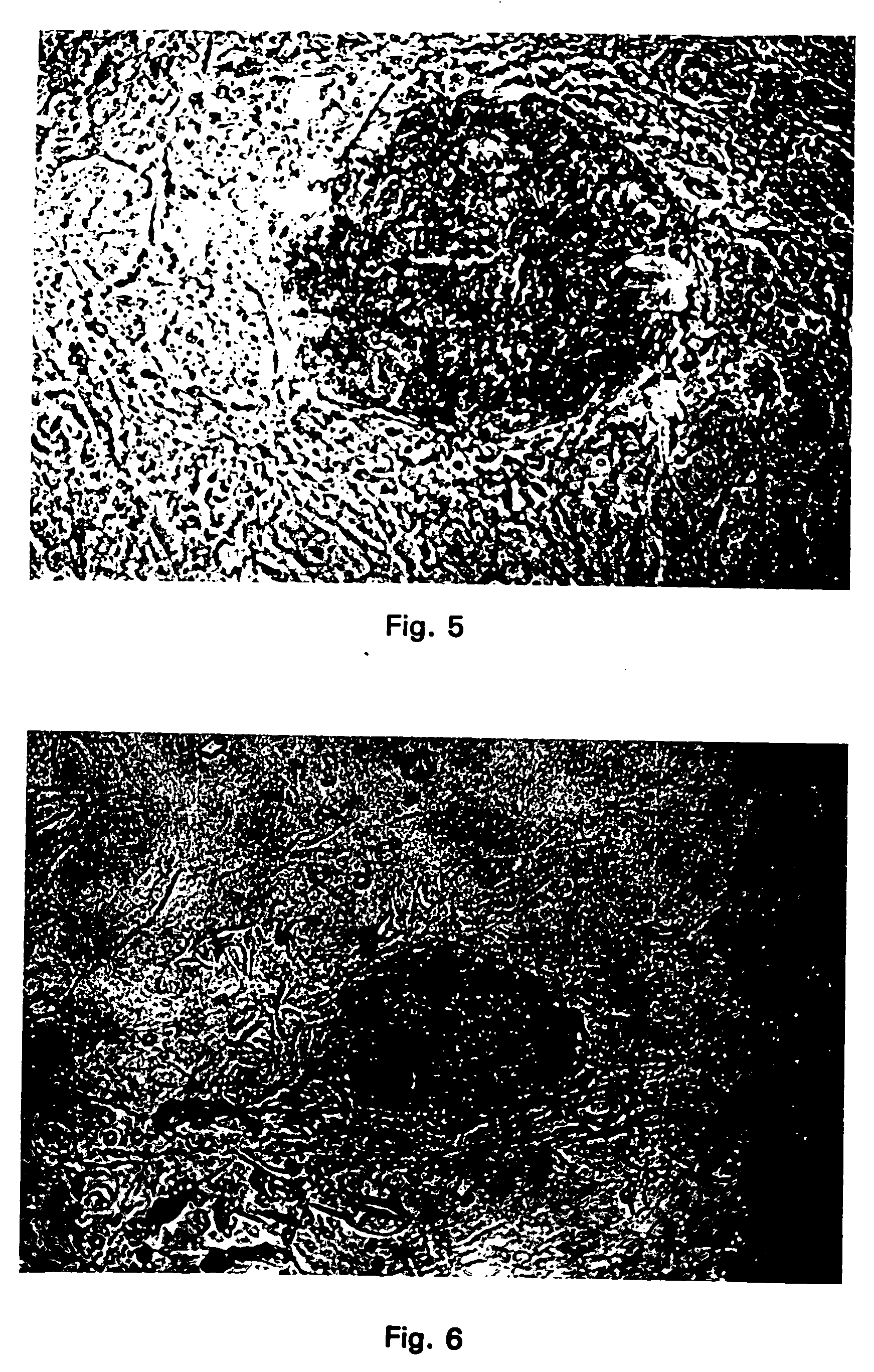
[0193] CICM cells obtained from cattle according to Example 1 were used for insertion of heterologous DNAs followed by selection in vitro for cells that stably expressed the heterologous DNAS. In this example the cytomegalovirus promoter and a fusion gene consisting of both the beta-galactosidase gene and the neomycin phosphotransferase gene (beta-GEO) were used. Microinjection of the gene construct into the CICM cells followed the same procedure as described in Example 2 for both the bovine and the porcine CICM cells. The CICM cells were propagated on primary mouse embryonic fibroblast cells derived from a line of transgenic mice (commercially available from Jackson Labs) that have the neomycin phosphotransferase gene in their genome. The day after the CICM cells were microinjected they were placed in selection growth medium containing the selection compound G418 (genticin). This drug kills any cell that is not expressing the neomycin phosphotransferase gene.
[0194] The transgenic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com