Human albumin animal models for drug evaluation, Toxicology and immunogenicity studies
a human albumin and animal model technology, applied in the field of human albumin animal model for drug evaluation, toxicology and immunogenicity studies, can solve the problem of extremely disappointing results when used in human trials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Injection of Albumin into NAR Rats
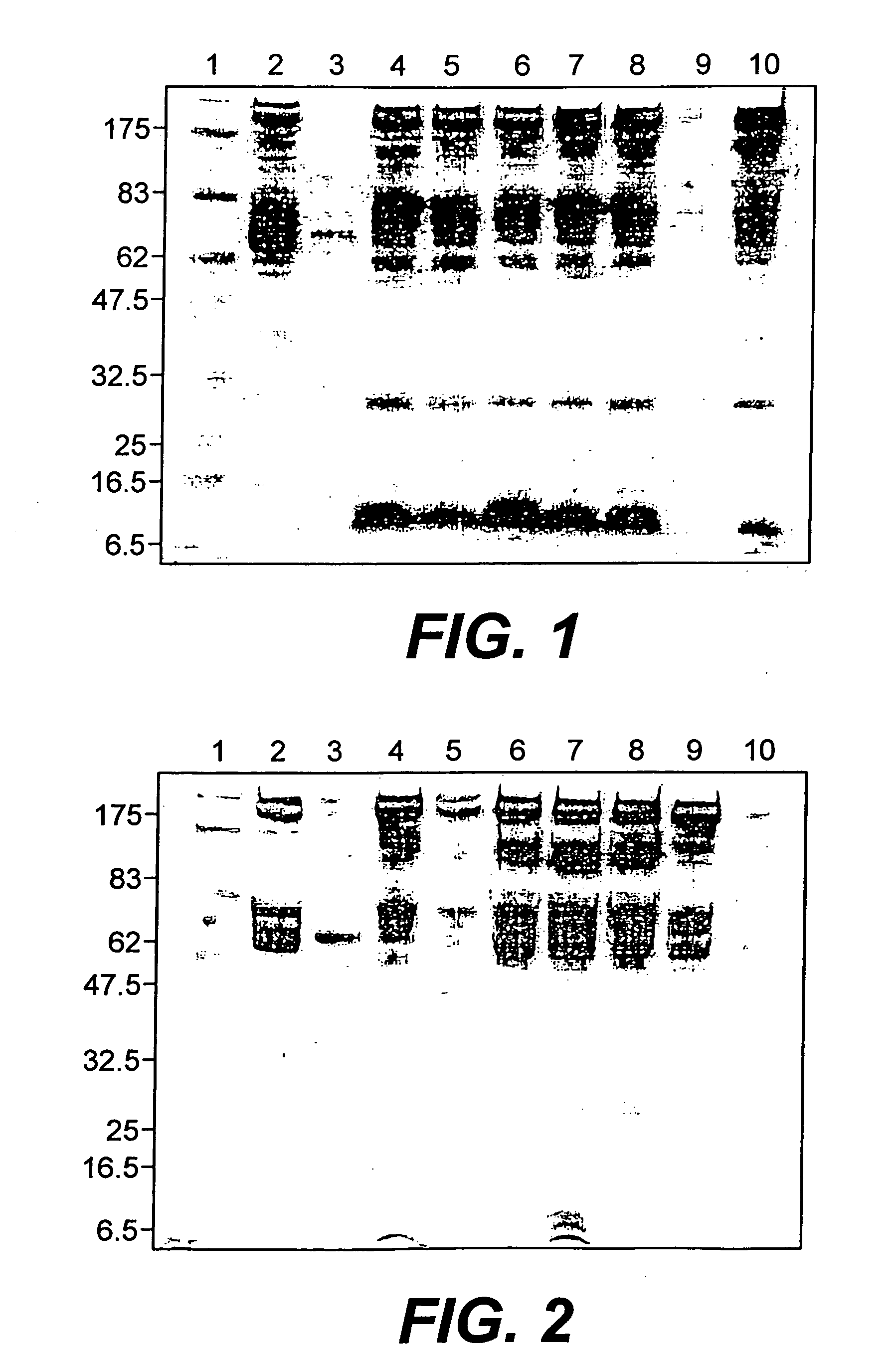
[0035] In accordance with the present invention, a natural mutant rat which does not express or contain its own serum albumin, namely an NAR rat identified as ODNAR10, was utilized as an animal model in accordance with the present invention by injecting the animal with suitable amounts of human serum albumin. In this example, the NAR was given intravenous albumin injection / infusions by initially injecting relatively small amounts of HSA solution into the animal (1 mL). The amount of blood administered to this initial NAR rat was monitored over a 25 day period and this monitoring showed that a significant amount of human albumin had accumulated in the rat's system during this time, as shown by the chromatograph of FIG. 1, wherein column 10 represents the blood sample obtained from ODNAR10. By the end of the treatment (when the animal expired during anesthesia), ODNAR10 had received a total of 2.125 g HSA administered over a 25 day period (Table I)....
example 2
Transgenic NAR Rats
[0039] In accordance with the present invention, transgenic animal models were prepared which were transfected with the gene for human serum albumin. In this example, first generation NAR rat pups were sent to TOSK for injection of the HSA gene within their proprietary STEALTHGENE vector. Pups were sent to TOSK between the weights of 80 and 120 g, as per their instructions, due to their procedure being most amenable to this age and weight of rat. From the breeding program we have instigated for NAR rats, we have learned that females need to be on the order of 230 g before they conceive reliably. As of today, our heaviest females returned from TOSK have been transfected with the gene for human serum albumin and are 185.5 g and 190 g and growing at a rate of about 15 g every 3 to 4 days. In accordance with the invention, these animals are thus suitable as models for animal testing, and because they have been genetically transformed, such animals will be useful in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| osmotic blood pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com