Method for treating a condition with neural progenitor cells derived from whole bone marrow
a neural progenitor cell and whole bone marrow technology, applied in the direction of genetically modified cells, skeletal/connective tissue cells, peptide/protein ingredients, etc., can solve the problems of determining precisely how or why the myeloid is rare, incomplete description of hematopoiesis, and affecting the survival rate of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation and Preparation of Neural Progenitor Cells
[0039] Whole bone marrow was harvested from the femurs of adult Fisher rats between 16 and 24 weeks of age. Cultures were plated on poly-D-lysine coated 24 well plates at a density of 106 cells per well. The cells were cultured in serum-free Dulbecco's modified Eagle medium (DMEM) / F-12 medium supplemented with B27 (obtained from Gibco BRL; Gaithersburg, Md.), 20 ng / ml FGF-2 and 20 ng / ml EGF (both available from Sigma Chemical Co.; St. Louis, Mo.; hereinafter “Sigma”), along with penicillin and streptomycin (both available from Omega Scientific, Inc.; Tarzana, Calif.).
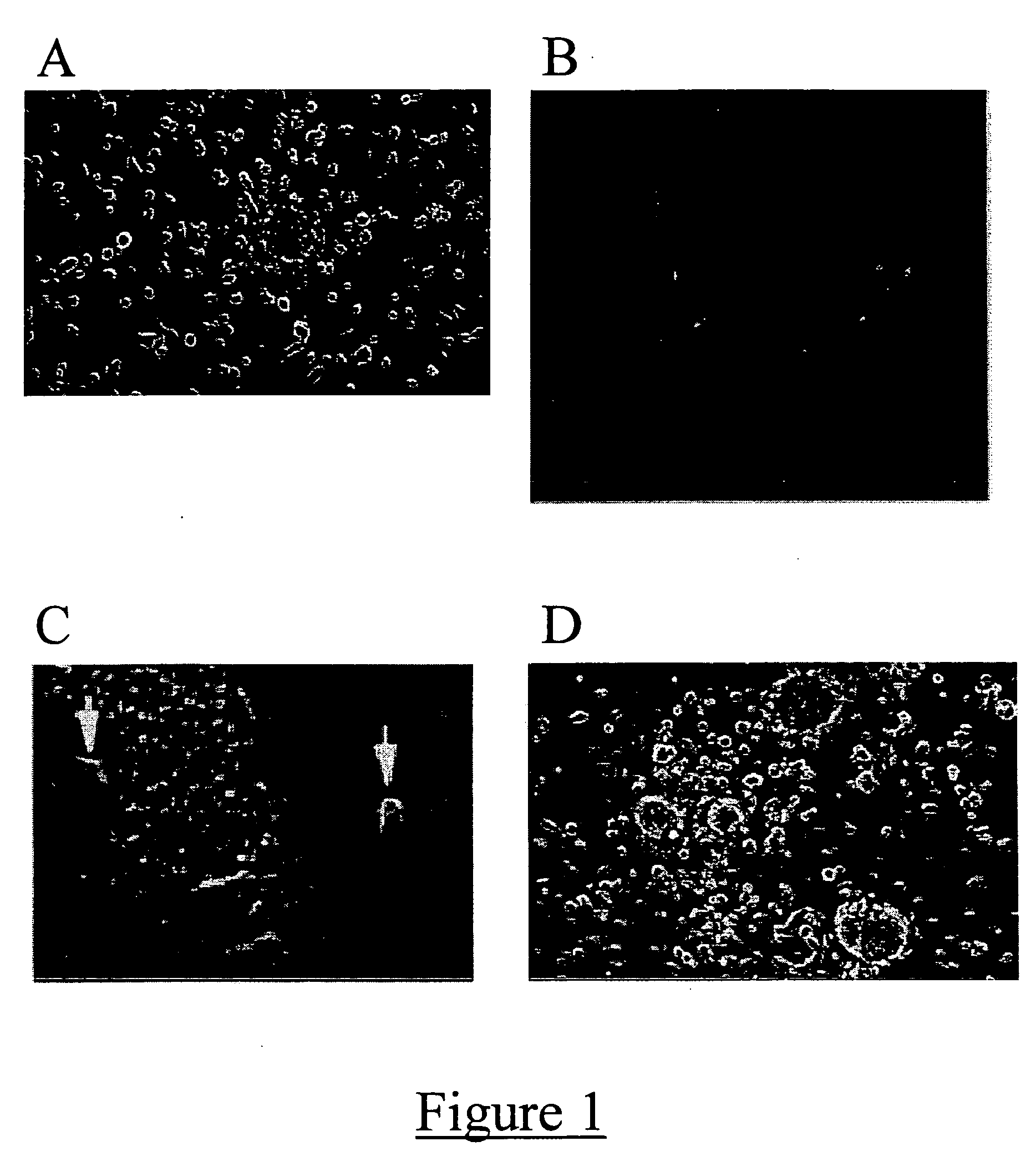
[0040] After four days in culture, numerous floating spheres of between about 10 to about 100 cells were distinctly visible separate from an underlying adherent monolayer (FIG. 1A). These spheres were collected and sub-cultured separately (FIG. 1B). The cellular aggregates continued to expand and the rate of proliferation remained stable even after multiple disassoci...
example 2
Gene Transfer into Neural Progenitor Cells Utilizing Replication-Deficient Adenoviral Vectors
[0043] Type 5 replication-deficient adenoviral vectors bearing either the reporter gene for β-galactosidase or the gene for the cytokine IL-12 were used to infect neural progenitor cells in vitro. 24 hours following infection, successful gene transfer was confirmed using X-gal staining (X-gal Staining Assay Kit available from Gene Therapy Systems, Inc.; San Diego, Calif.) for α-galactosidase-bearing adenoviral-infected progenitor cells, and an IL-12 Enzyme Linked Immunosorbent Assay (“ELISA” kit available from BD Pharmingen; San Diego, Calif.) for IL-12 gene-bearing adenovirus-infected progenitor cells.
[0044] Successful gene transfer of β-galactosidase was confirmed by positive staining for the X-gal and β-galactosidase-generated blue precipitate in the β-galactosidase-bearing adenovirus-infected progenitor cells (FIG. 5). Successful gene transfer of IL-12 was confirmed by the positive pho...
example 3
Gene Transfer into Neural Progenitor Cells Utilizing a Double-Mutated Herpes Simplex Virus Type I
[0045] A herpes simplex type I virus deleted for the genes encoding the latency activated transcript (LAT) and gamma 34.5 genes (virus denoted DM33) was utilized. The virus contained the gene for GFP under the control of the powerful LAT promoter, and was therefore able to confer constitutive expression of GFP into any successfully infected cell. This vector was used to infect neural progenitor cells in vitro. 72 hours after infection, successful gene transfer was confirmed by viewing GFP expression under a fluorescent light microscope (FIG. 6).
[0046] GFP expression was visible in neural progenitor cells 72 hours following infection with DM33. This confirmed the ability to successfully utilize herpes simplex type I for gene transfer to neural progenitor cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com