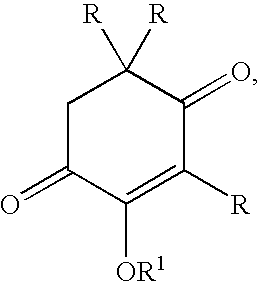
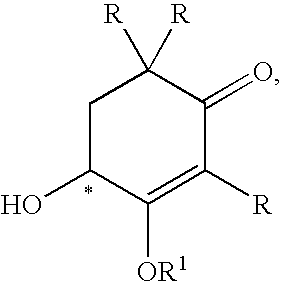
Methods for the synthesis of astaxanthin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of (R)-4-hydroxyisophorone (R)-116
[0154]
[0155] All solvents were free of O2. And the reactions were done under N2. Benzeneruthenium (II) dimer (19.72 g, 39.42 mmol, 0.4 mol %) and (1R, 2S)-(−)-norephedrine (99%) (24.14 g, 159.67 mmol, 1.62 mol %) were dissolved in a 12 L three-necked flask containing 2-propanol (7.5 L). After stirring the red solution for 45 min at 80° C., the heat was removed. It was transferred to a 50 L three-necked flask containing 2-propanol (28 L). 109 (1500 g, 9.86 mol) and 0.1 M potassium hydroxide in 2-propanol (3945 ml, 0.0.395 mol, 4 mol %) were added. After 3 h (TLC showed the reaction was done), the red solution was filtered through a short silica gel pad and the filtrate was evaporated to dryness to obtain solids (about 1600 g). After five times recrystallization from iPr2O (500 ml×5), 912 g of (R)-116 was obtained. The yield: 60%. 1H NMR (CDCl3, 300 MHz): δ 1.02 (s, 3H), 1.07 (s, 3H), 1.97 (s, 1H), 2.04 (t, J=1.2 Hz, 3H), 2.21 (d, J=16.3 H...
example 2
Preparation of (1R, 4S)-2,6,6-trimethyl-2-cyclohexen-1,4-diol (1R,4S)-118
[0156]
[0157] To a solution of L-Selectride (5674 mL, 1 M in THF, 1.25 equiv), a solution of compound (R)-116 (700 g, 4.54 mol, 1 equiv) in THF (3000 mL) was added dropwise at −78° C. After stirring for 1.5 h, the mixture was sequentially treated with H2O (600 mL), 4N NaOH (1450 mL). After extractions with AcOEt (500 ml×5) and the combined organic phase was dried and concentrated. To the residue was charged 3000 mL of hexanes, then the mixture was filtered. The solid was washed with hexanes (200 mL×3). The solid crude product was purified by flash chromatography using Hexanes / AcOEt (3 / 1) as an eluent. 645 g of compound (1R,4S)-118 was obtained (yield: 91%). Recrystallized from 1000 ml of EtOAc to obtain 504 g (70%) of (1R,4S)-118. 1H NMR (CDCl3, 500 MHz): δ 0.86 (s, 3H), 1.02(s, 3H), 1.45 (dd, J=12.8,9.5 Hz, 1H), 1.67 (ddt, J=12.8, 6.3, 1.1 Hz, 1H), 1.84 (t, J=1.7 Hz, 3H), 3.34 (s, 1H), 4.18 (m, 1H), 5.54 (br s,...
example 3
Preparation of (1R, 4S)-4-tert-Butyidimethylsilyloxy-2,6,6-trimethyl-2-cyclohexen-1-ol (1R, 4S)-120a
[0158]
[0159] A mixture of enantiomerically pure (1R, 4S)-118 (1000 g, 6.40 mol), TBDMSCI (1194 g, 7.68 mol, 1.2 eq) and imidazole (566.37 g, 8.32 mol, 1.3 eq) in DMF (9 L) was stirred at room temperature for 1 hr and 20 min. Water (2 L) was added, aqueous phase was extracted with diethyl ether (2000 ml×3). The combined organic layer was dried over Na2SO4. After concentration, the crude product (1R, 4S)-120a was subjects to next step without further purification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com