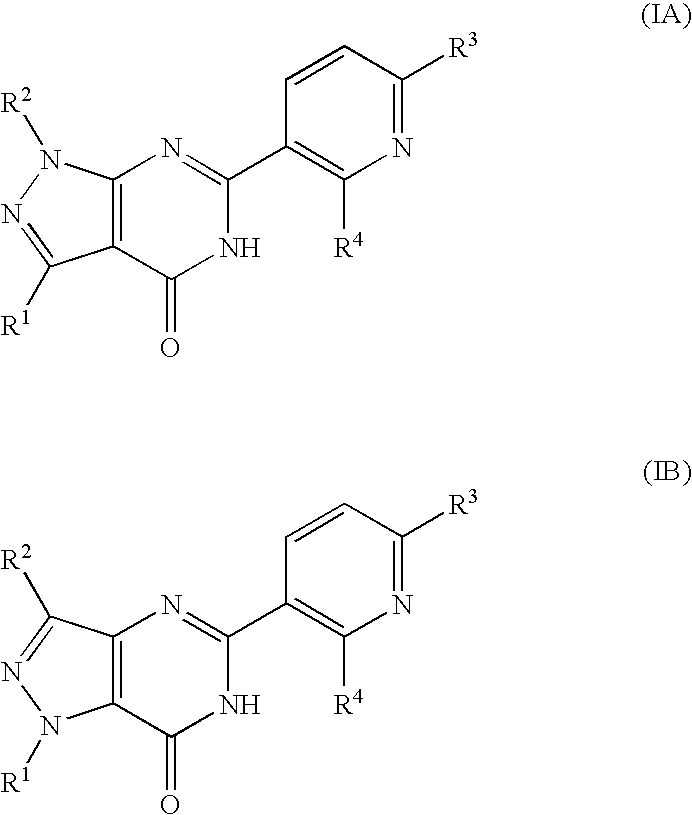
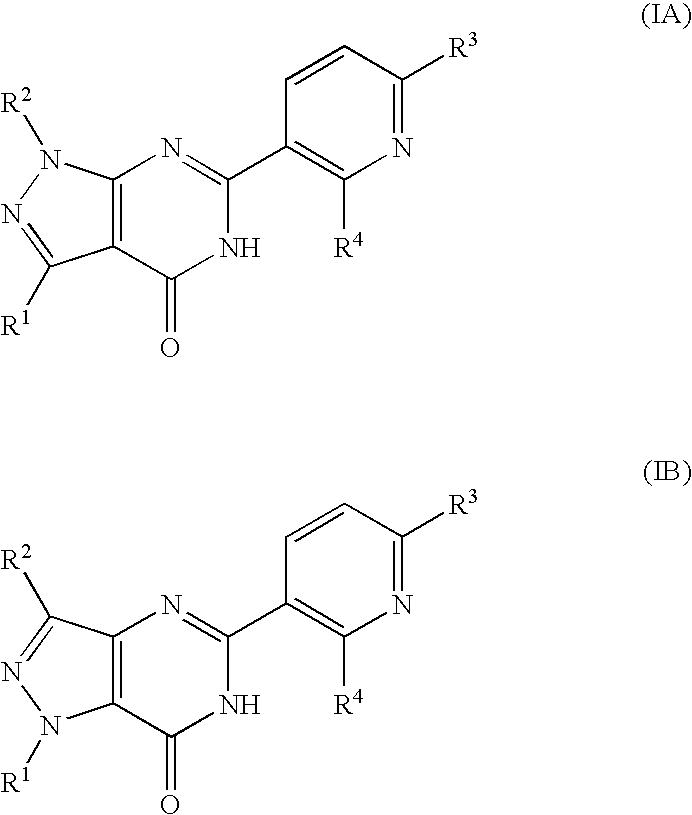
Pyridinylpyrazolopyrimidinone derivatives as pde 7 inhibitors
a technology of pyrazolopyrimidinone and derivatives, which is applied in the direction of antibacterial agents, immunological disorders, drug compositions, etc., can solve the problem that no curative medicines with pde 7 inhibiting effect as main pharmacological mechanism have developed up to now, and achieve significant pde 7 inhibiting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0106] The present invention is illustrated in more detail by way of the following Biological Test and Examples, but it is to be noted that the present invention is not limited by those Examples in any way.
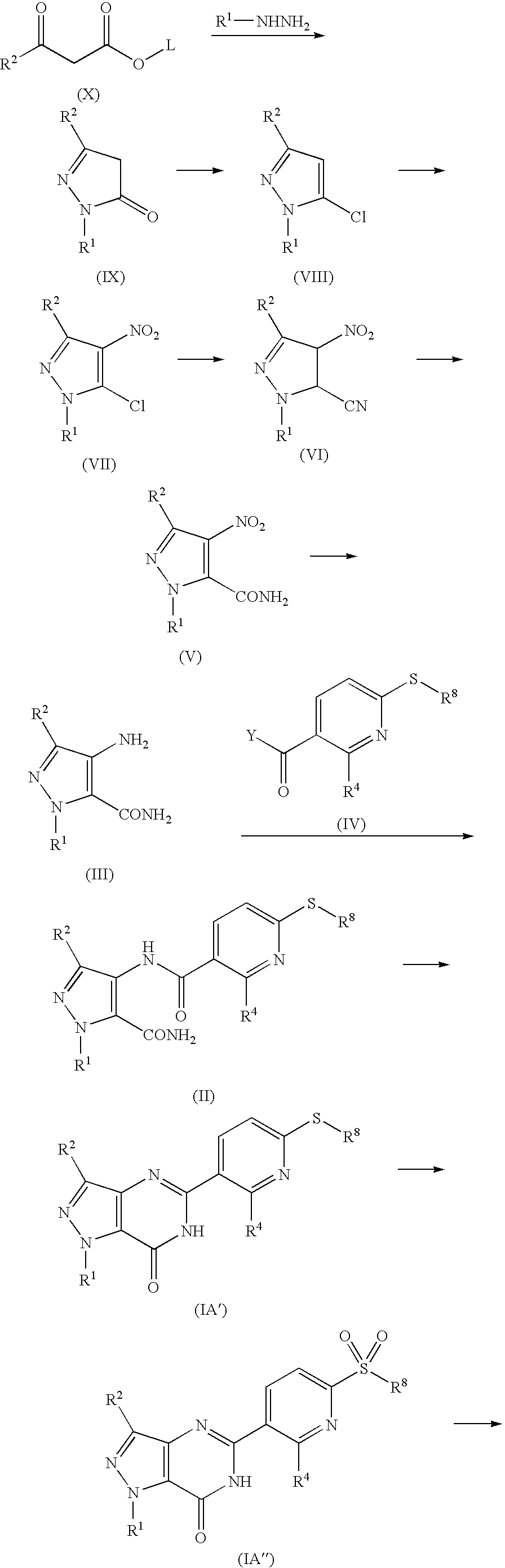
[0107] The synthesis of the compounds of the present invention and intermediate compounds to be used in the synthesis are illustrated in the Example mentioned later. Further, the physicochemical data and chemical structure of the compounds and intermediate compounds obtained by the Examples are summarized in the Tables mentions later.
[0108] The compound numbers in the Examples are identical to those in the Tables.
[0109] The PDE 7 (phosphodiesterase VII) inhibiting effect of the compounds of the present invention obtained in the later mentioned Examples was evaluated by mean of the following Biological Tests.
manufacturing examples and examples
[0134] The synthesis of the compounds of the present invention is illustrated in the following Examples.
[0135] The physicochemical data and chemical structure of the compounds are summarized in the Tables mentions later. The compound numbers in the Examples are identical to those in the Tables.
example 1
2-Cyclohexyl-5-Methyl-2,4-Dihydro-3H-Pyrazol-3-One
[0136] A mixture solution of 14.5 mL(0.134 mol) of methyl acetoacetate and 20.2 g (0.134 mol) of cyclohexylhydrazine hydrochloride was stirred for 2 hours at 120° C., and the mixture was cooled. Then, the reaction mixture was neutralized with 30 mL of 4M-sodium hydroxide solution and extracted with ethyl acetate. The organic layer was washed with water and saturated saline solution and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the resulting residue was treated with hexane. The resulting precipitate was collected to give 19.0 g (79%) of the titled compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com