Expandable intraluminal graft
a technology expanding graft, which is applied in the field of expanding intraluminal graft, can solve the problems of inability to change the expansion size of the graft, no effective control over the final, and the foregoing structure has one major disadvantage in common, so as to prevent recurrence of stenoses and prevent erosion of the body passageway
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
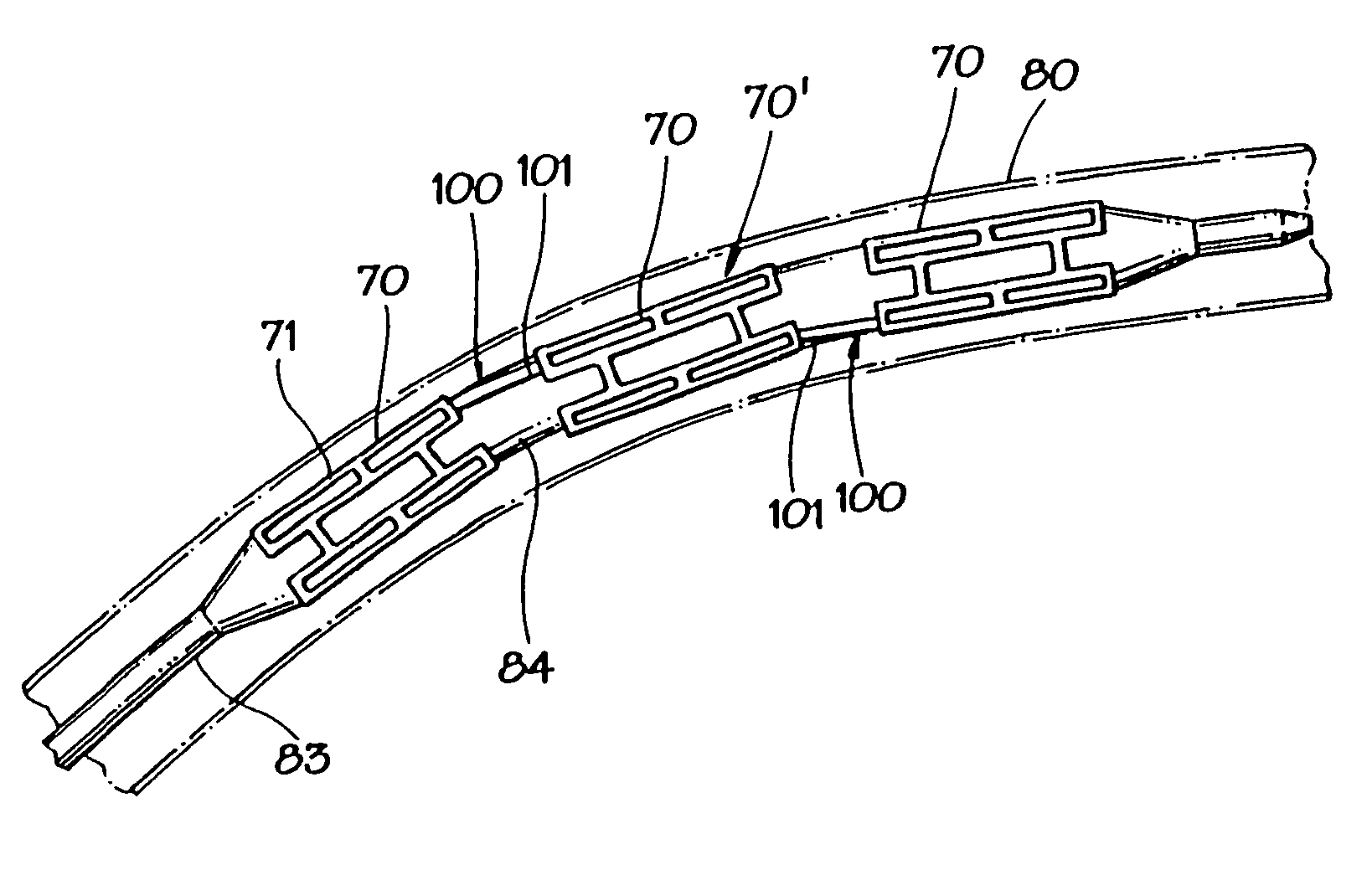
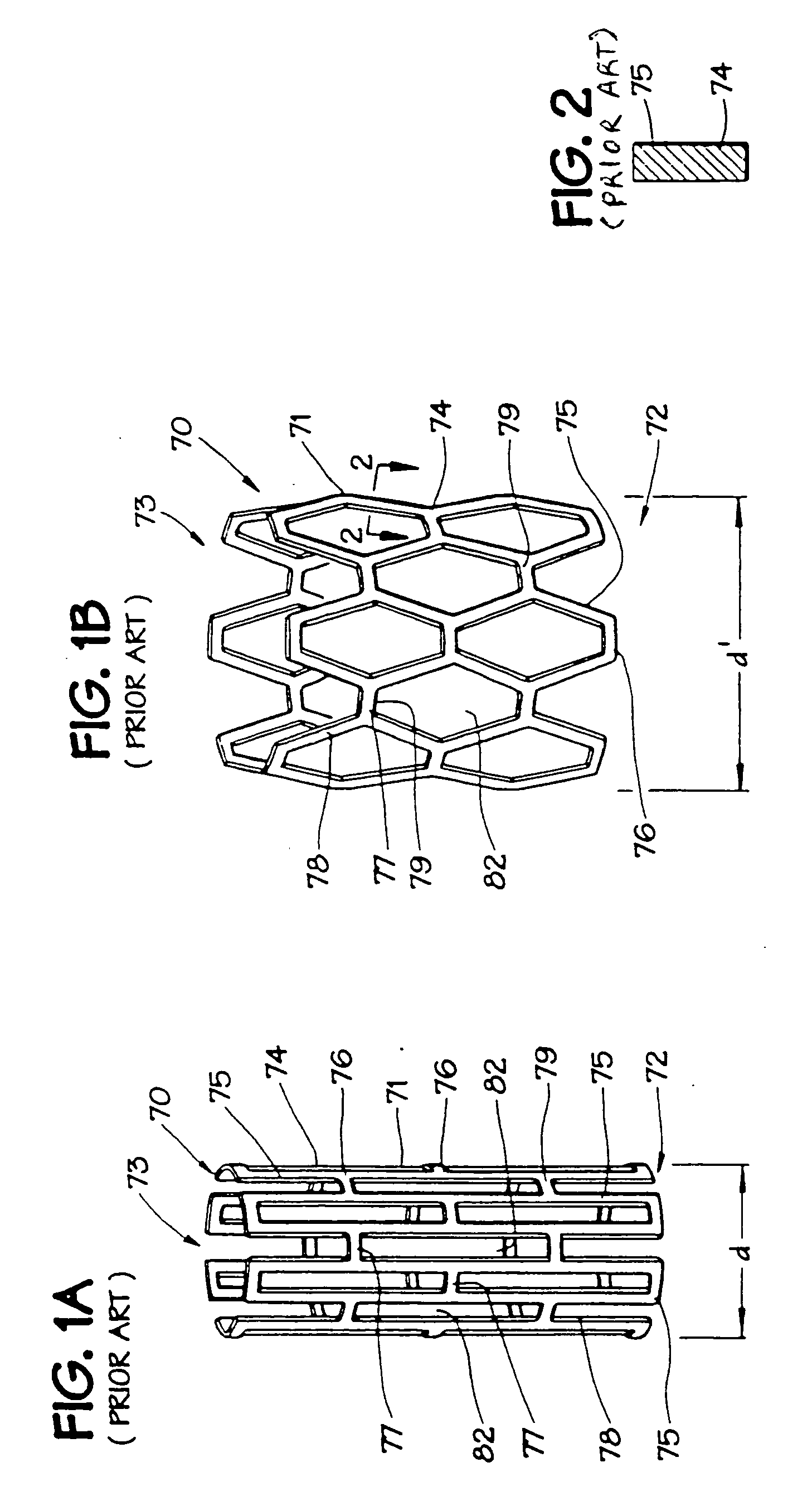
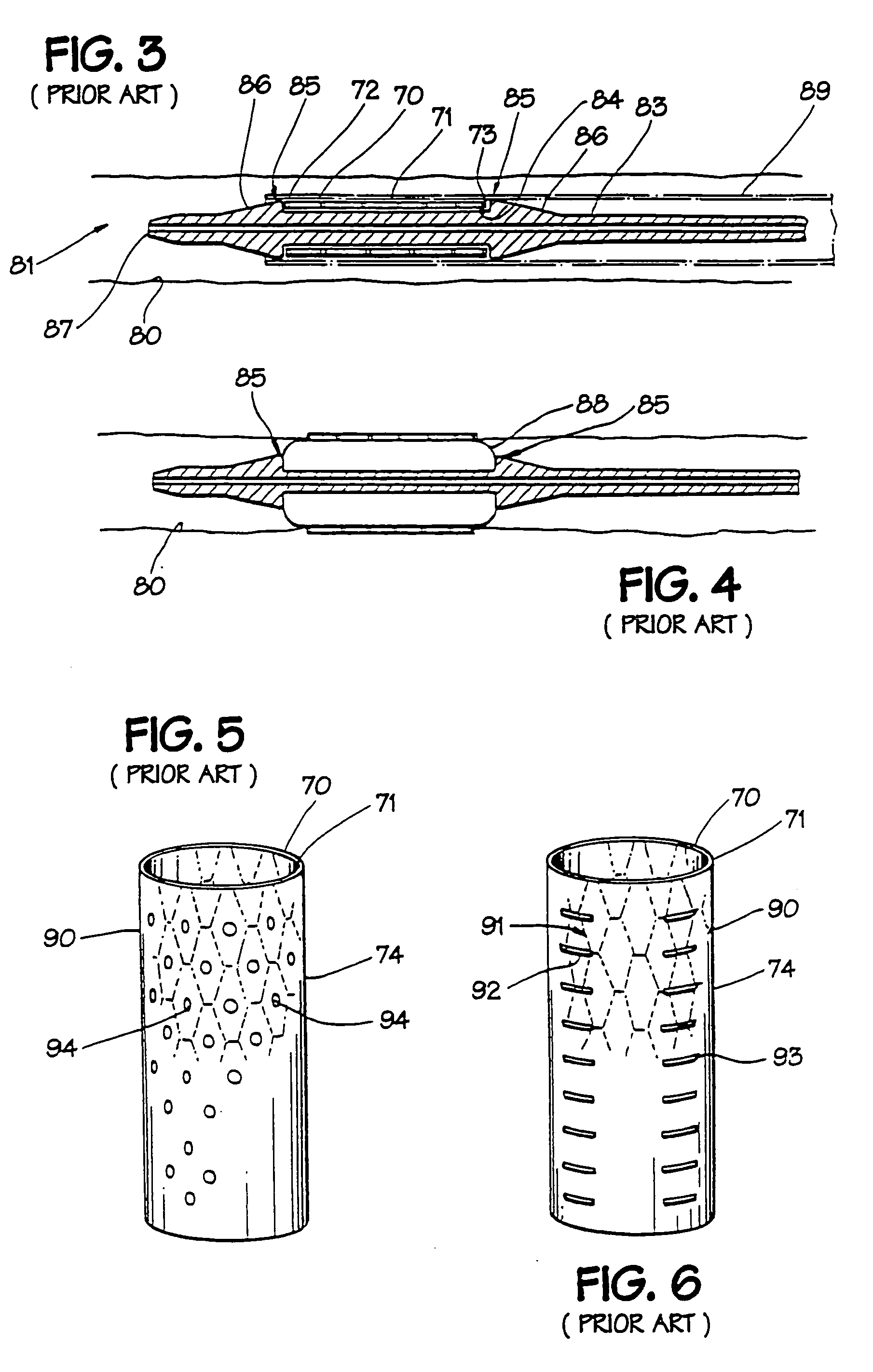
[0024] In FIGS. 1A and 1B, an expandable intraluminal vascular graft, or expandable prosthesis for a body passageway, 70 is illustrated. It should be understood that the terms “expandable intraluminal vascular graft” and “expandable prosthesis” are interchangeably used to some extent in describing the present invention, insofar as the methods, apparatus, and structures of the present invention may be utilized not only in connection with an expandable intraluminal vascular graft for expanding partially occluded segments of a blood vessel, or body passageway, but may also be utilized for many other purposes as an expandable prosthesis for many other types of body passageways. For example, expandable prostheses 70 may also be used for such purposes as: (1) supportive graft placement within blocked arteries opened by transluminal recanalization, but which are likely to collapse in the absence of an internal support; (2) similar use following catheter passage through mediastinal and othe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com