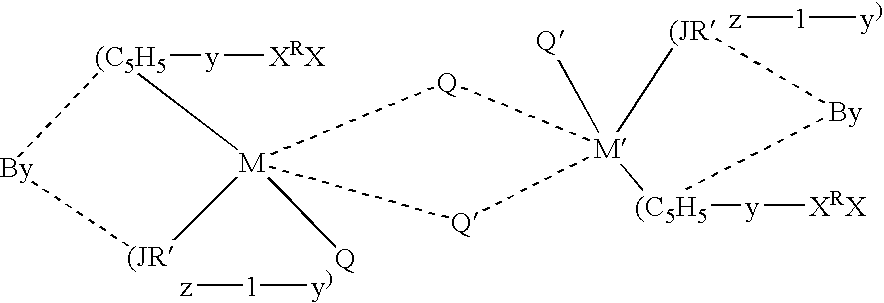
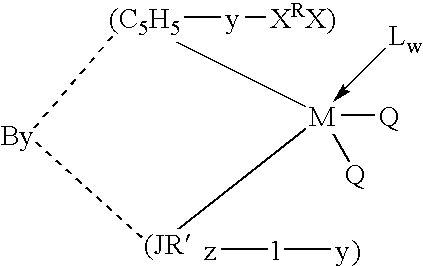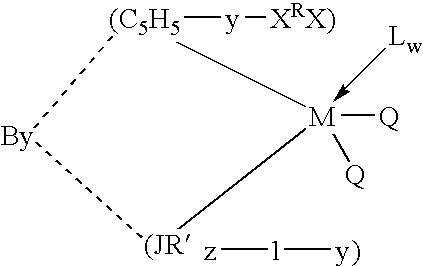Olefin polymerization catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0045] In the examples which illustrate the practice of the invention the analytical techniques described below were employed for the analysis of the resulting polyolefin products. Molecular weight determinations for polyolefin products were made by Gel Permeation Chromatography (GPC) according to the following technique. Molecular weights and molecular weight distributions were measured using a Waters 150 gel permeation chromatograph equipped with a differential refractive index (DRI) detector and a Chromatix KMX-6 on-line light scattering photometer. The system was used at 135° C. with 1,2,4-trichlorobenzene as the mobile phase. Shodex (Showa Denko America, Inc.) polystyrene gel columns 802, 803, 804 and 805 were used. This technique is discussed in “Liquid Chromatography of Polymers and Related Materials III”, J. Cazes editor, Marcel Dekker, 1981, p. 207 which is incorporated herein by reference. No corrections for column spreading were employed; however, data on generally accept...
examples a -
EXAMPLES A-L OF GROUP IV B TRANSITION METAL COMPONENTS
example a
[0048] Compound A: Part 1. Me4HC5Li (10.0 g, 0.078 mol) was slowly added to a Me2SiCl2 (11.5 ml, 0.095 mol, in 225 ml of tetrahydrofuran (thf) solution). The solution was stirred for 1 hour to assure complete reaction. The thf solvent was then removed via a vacuum to a cold trap held at −196° C. Pentane was added to precipitate out the LiCl. The mixture was filtered through Celite. The solvent was removed from the filtrate. Me4HC5SiMe2Cl (15.34 g, 0.071 mol) was recovered as a pale yellow liquid.
[0049] Part 2. Me4HC5SiMe2Cl (10.0 g, 0.047 mol) was slowly added to a suspension of LiHN-t-Bu (3.68 g, 0.047 mol, ˜100 ml thf). The mixture was stirred overnight. The thf was then removed via a vacuum to a cold trap held at −196° C. Petroleum ether (−100 ml) was added to precipitate out the LiCl. The mixture was filtered through Celite. The solvent was removed from the filtrate. Me2Si(Me4HC5)(HN-t-Bu) (11.14 g, 0.044 mol) was isolated as a pale yellow liquid.
[0050] Part 3. Me2Si(Me4HC5)(H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com