Binuclear metallocene compound and its preparation and application in olefinic polymerization
A technology of metallocene compounds and compounds, applied in the field of binuclear metallocene compounds, can solve problems such as poor processability, narrow polyolefin molecular weight distribution, and increased processing difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
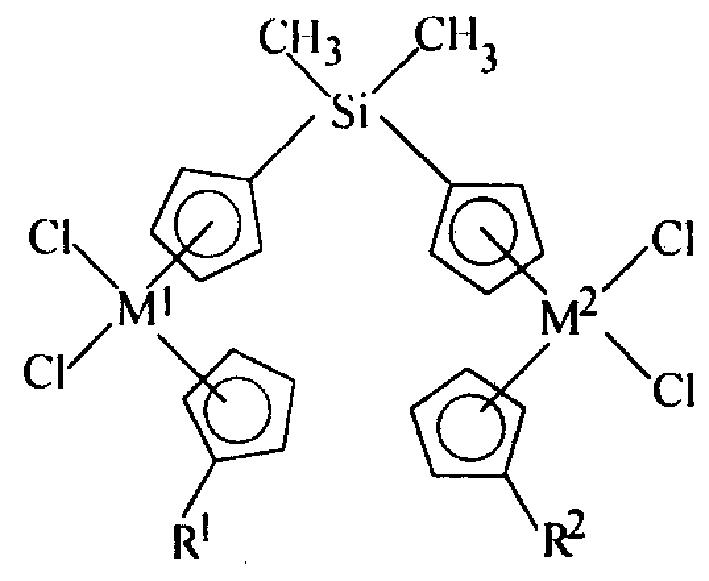
[0016] The preparation method of compound (I) can refer to T Ushioda, et al, J. Organomet. Chem, 518 (1996) 155-166. The detailed preparation steps are as follows: the structural expression is The silane compound ligand is dissolved in n-hexane, and n-butyllithium is added dropwise at a temperature of -30 to 20°C, preferably -10 to 0°C. The molar ratio to n-butyllithium is 1:2.0-2.5. After the dropwise addition is completed, it is naturally raised to room temperature and reacted for 8-15 hours. The solvent was removed by filtration, and the solvent was sucked dry under reduced pressure. This gives the compound of formula (II) as the lithium salt of the Si-bridged biscyclopentadienyl ligand. Toluene was added to the solid lithium salt to obtain a white turbid liquid, namely the suspension of the above lithium salt. Add CpM at room temperature 1 Cl 3 DME, wherein DME is ethylene glycol dimethyl ether, Cp is cyclopentadienyl, M 1 for zirconium. CpM 1 Cl 3 ·The molar ra...
example 1
[0021] The preparation formula is Ligand Compound A.
[0022] Will 5 grams (0.0266mol) was dissolved in 80 ml of n-hexane, and 0.0532mol of n-butyllithium was added dropwise in an ice-water bath. After the dropwise addition, the ice bath was removed, and the mixture was naturally raised to room temperature and reacted overnight. The solvent was removed by filtration and dried under vacuum. 150 ml of toluene was added to obtain a white cloudy liquid. Add 9.36 g of cyclopentadienyl zirconium trichloride ethylene glycol dimethyl ether (CpZrCl 3 ·DME), stirred at 25°C for 24 hours. After centrifugation, the residue was extracted twice with 50 ml of dichloroethane, the centrifuged liquid and the extract were combined, concentrated until a solid appeared, and left standing at -20°C for 24 hours to obtain 1.933 g of pale yellow needle-like crystals, which was compound A. Yield 19.2 wt%.
example 2
[0024] The preparation formula is Ligand compound B
[0025] Will 5 grams (0.0266 mol) was dissolved in 80 ml of n-hexane, and 0.0532 mol of n-butyllithium was added dropwise under an ice-water bath. After the dropwise addition, the ice bath was removed, and the mixture was naturally raised to room temperature, and reacted overnight. The solvent was removed by filtration and dried under vacuum. 5.30 g of a white powdery solid was obtained, which was compound B, and the yield was 99.6% by weight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com