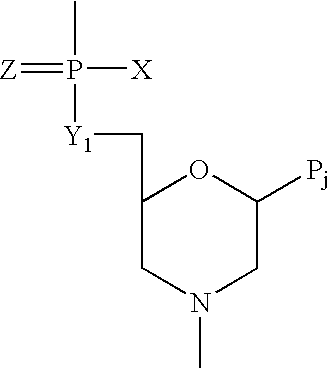
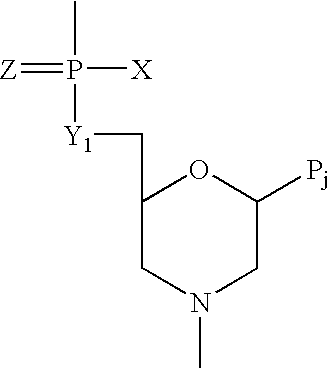
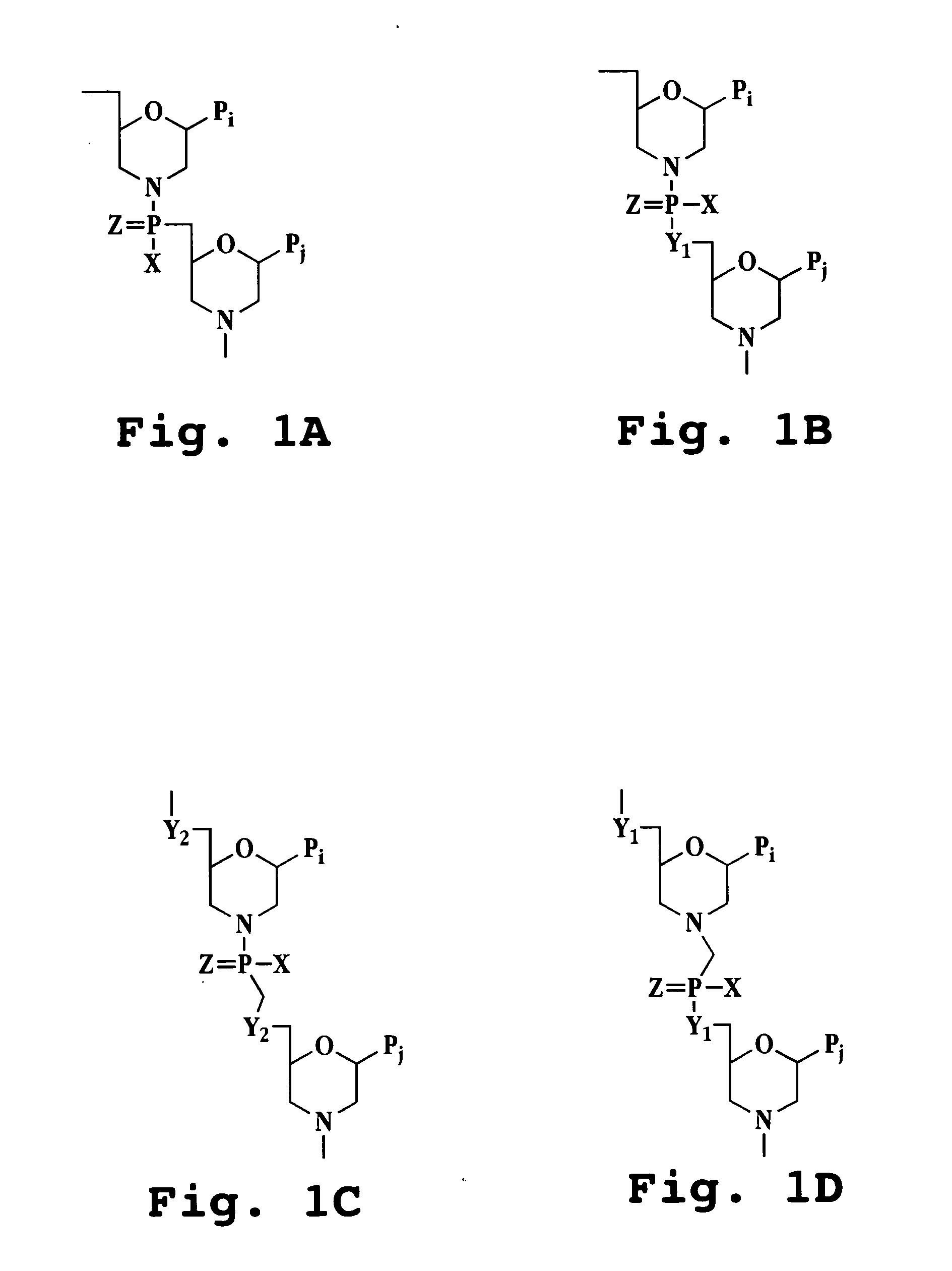
Antisense antiviral compounds and methods for treating a filovirus infection
a technology of antiviral compounds and filovirus, applied in the field of antiviral compounds and methods for treating filovirus infections, can solve the problems of ebola virus being considered a significant world health threat, unable to develop an effective treatment for ebola virus, and no effective antiviral therapy available to treat an infection by any of these viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antiviral Efficacy of Ebola Virus-Specific PMOs in Rodents
[0194] To determine the in vivo efficacy of the Ebola virus-specific PMOs, the survival of mice treated with 500 μg doses of the individual PMOs (VP24-AUG, L′-AUG and VP35′-AUG, SEQ ID NOs:34, 17 and 22, respectively) at 24 and 4 hours before challenge with 1000 plaque-forming units (pfu) of mouse-adapted Ebola virus was determined. The VP35′-AUG, VP24-AUG and L′-AUG PMOs exhibited a wide range of efficacy against lethal EBOV infection and the VP35′-specific PMO provided nearly complete protection (FIG. 11A). Next, we performed a dose response experiment with the VP35 PMO and found that reducing the dose of the PMO from 1,000 to 100 μg reduced the efficacy substantially (FIG. 11B). Hence, to further enhance efficacy, we decided to use a combination of all three PMOs. This combination of PMOs administered 24 and 4 h before lethal Ebola virus challenge resulted in robust protection and showed substantial enhancement in protect...
example 2
Antiviral Efficacy of Ebola Virus-Specific PMOs in Non-Human Primates
[0197] Based on the encouraging results both in vitro and in rodents, a trial in nonhuman primates was performed. Four rhesus monkeys were treated with PMO from two days prior to Ebola virus infection through day 9 of the infection. The naive control monkey in this experiment received no treatment and succumbed to Ebola virus infection on day 10 as shown in FIG. 16A. Of 12 rhesus monkeys that have been infected in the inventors' laboratory with the same seed stock of virus, all died of Ebola virus between days 7 and 10 as shown in FIG. 16A. One of the PMO-treated monkeys succumbed to the infection on day 10. A second PMO-treated monkey cleared the EBOV infection from its circulation between days 9 and 14, but was unable to recover from disease and died on day 16 as shown in FIGS. 16A and 16B. The two surviving monkeys had no symptoms of disease beyond mild depression until day 35, at which time they were euthanize...
example 3
Increased Antisense of Activity Using PMO with Cationic Linkages
[0199] Two PMOs were synthesized using cationic linkages for a subset of the oligomer linkages as shown in Sequence Listing for SEQ ID NOs:40 and 41. These oligomers incorporated the cationic linkage (1-piperazino phosphoramidate) shown in FIG. 2H at the positions indicated with a “+”. These two PMOs target the EBOV VP24 mRNA. A cell free translation assay was performed using the VP24:luciferase mRNA as the input RNA. PMO with and without cationic linkages were compared for their ability to inhibit luciferase expression and the results are shown in FIG. 16. Compared to the uncharged PMO with the same base sequence, the PMOs with between 6 and 8 cationic linkages demonstrated between 10 and 100-fold increased antisense activity in this assay.
[0200] Based on the experiments performed in support of the invention as described above in the Examples, efficacious anti-filovirus PMOs have been identified. The antiviral PMOs d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com