Method and apparatus for producing metal
a metal and metal technology, applied in the field of metal production methods, can solve the problems of high production cost, high product price, and only high-quality and high-priced metallic titanium, and achieve the effect of high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
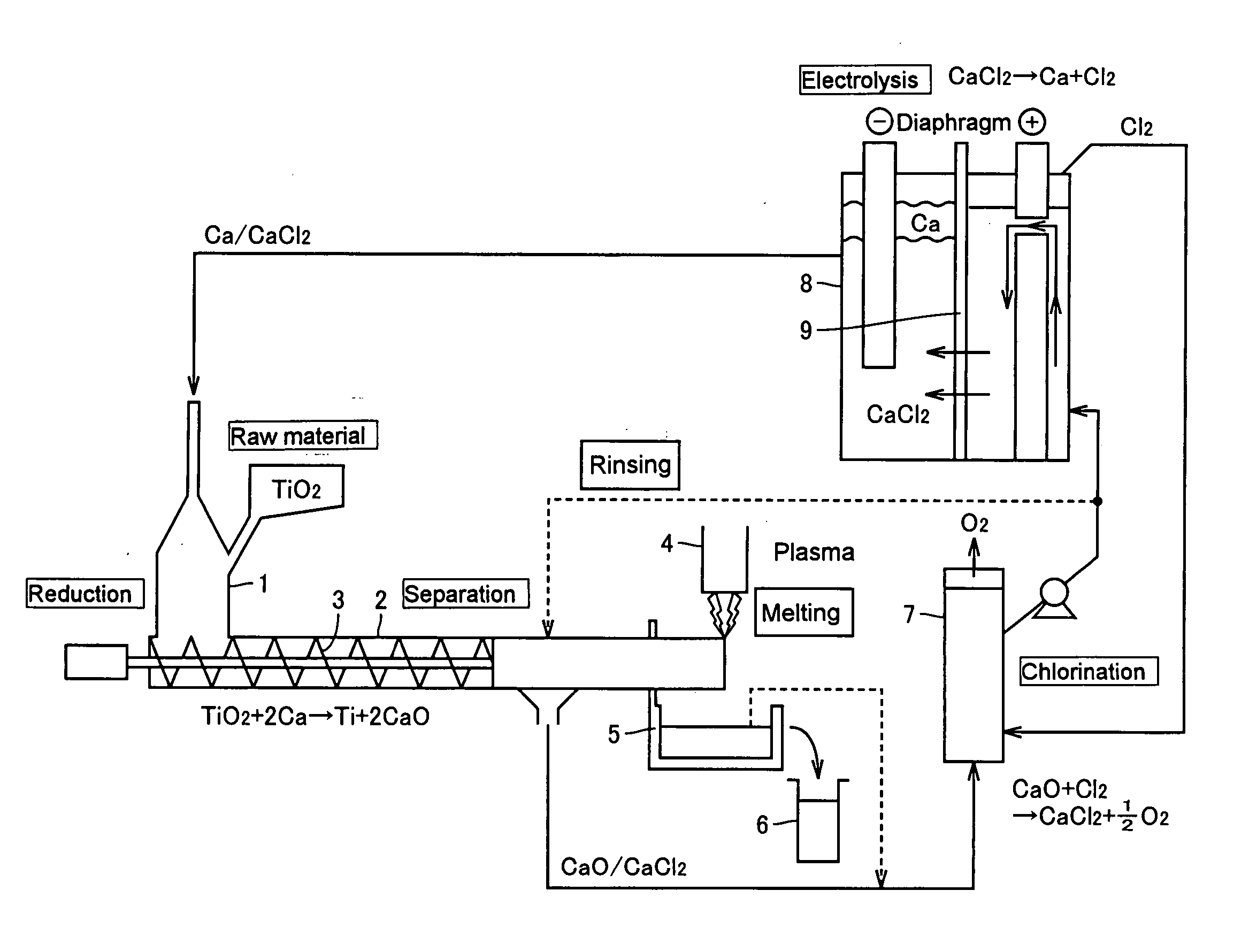
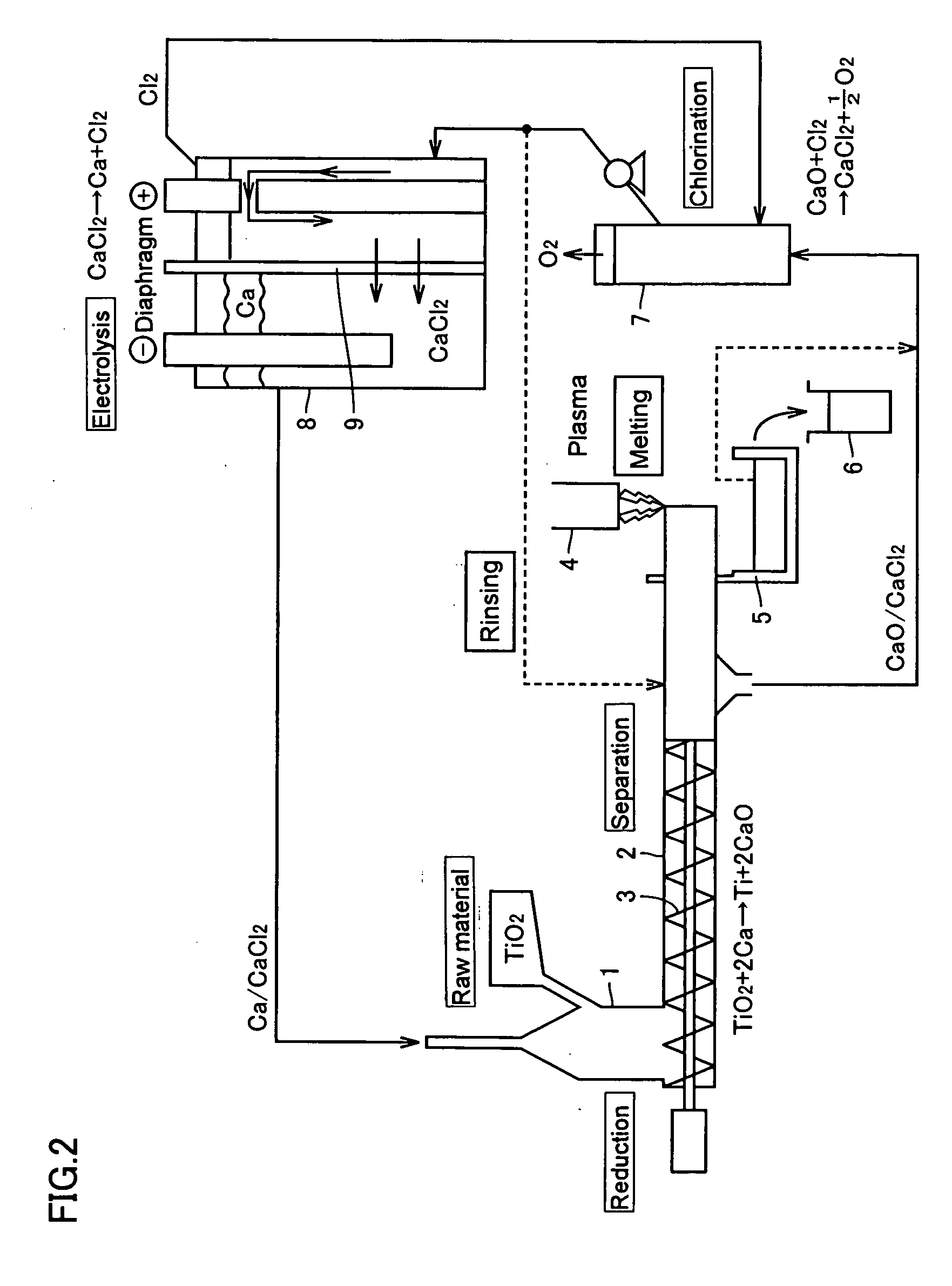
[0049]FIG. 2 is a drawing explaining the configuration of a titanium production apparatus according to the first embodiment of the present invention. In the first embodiment, the apparatus comprises a tower-shaped reduction chamber 1 and a horizontal separation means 2 connected to the lower part of the chamber 1. A CaCl2-based molten salt containing Ca is contained in the reduction chamber 1, and the raw material titanium oxide (TiO2) in the form of a powder is continuously introduced into the molten salt.
[0050] Thus, in the reduction chamber 1, TiO2 charged into the molten salt is reduced by Ca in the molten salt and metallic titanium (Ti) is formed and, at the same time, CaO is formed as a byproduct. Both the product Ti and the byproduct CaO settle and are separated from the molten salt and flow into the separation means 2 together with a certain amount of CaCl2.
[0051] A CaCl2-based molten salt containing Ca, together with the raw material titanium oxide, is additionally suppli...
second embodiment
[0060]FIG. 3 is a drawing explaining the configuration of a titanium production apparatus according to the second embodiment of the present invention. The second embodiment differs from the above-mentioned first embodiment as shown in FIG. 2 in that part of the molten salt after completion of the chlorination treatment in the chlorination chamber 7 is sent to the electrolysis chamber 8, while almost whole of the remaining molten salt is returned to the reduction chamber 1 and that the transfer of Ca from the electrolysis chamber 7 to the reduction chamber 1 is carried out in the form of a metal. In the second embodiment, however, the transfer of Ca in the metal form to the reduction chamber 1 may be combined with the method comprising the step of transferring Ca being dissolved in CaCl2.
[0061] Therefore, in the electrolysis chamber 8, the molten salt from the chlorination chamber 7 is introduced into the anode side, and the Ca formed on the liquid surface on the cathode side, eithe...
third embodiment
[0063]FIG. 4 is a drawing explaining the configuration of a titanium production apparatus according to the third embodiment of the present invention. The third embodiment differs from the production apparatus according to the second embodiment as shown in FIG. 3, featured in that the chlorination chamber 7 is integrated with the reduction chamber 1. Otherwise, the configuration is substantially the same as that of the production apparatus according to the second embodiment.
[0064] The chlorination chamber 7 is laterally disposed being annexed to the vertical type reduction chamber 1 via a partition wall 10. In the reduction chamber 7, titanium oxide is introduced into the CaCl2-based molten salt within the chamber through a feeding tube 11 inserted into the CaCl2. The titanium formed from titanium oxide upon reduction with Ca in the CaCl2 settles on the bottom of the reduction chamber 1 and downwardly extracted and sent to the lower separation means 2.
[0065] The CaCl2 containing th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| cylindrical shape | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com