Fluidic medical devices and uses thereof
a technology of medical devices and fluids, applied in the field of fluid medical devices, can solve the problems of buffers that may leak from holding areas, mix with dry reagents, and become wet or hydrated, and achieve the effect of reducing optical interference and reducing the amount of optical cross-talk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
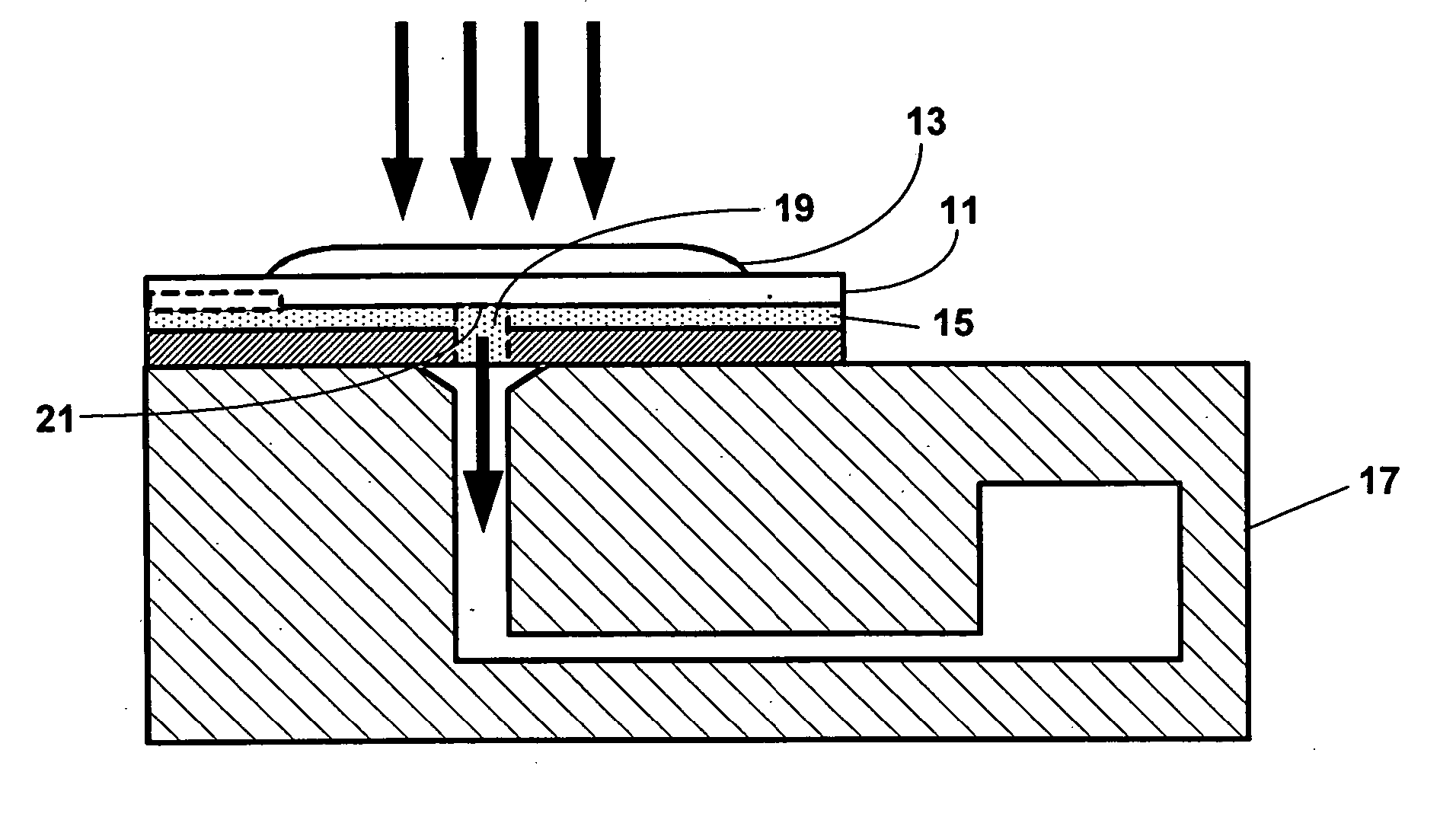
[0027] One aspect of the present invention is a system for detecting an analyte in a sample of bodily fluid. The subject system has one or more of the following components: a) a sample collection unit for introducing a biological fluid in fluid communication with a plurality of reaction sites, b) a plurality of reactant chambers carrying a plurality of reactants in fluid communication with said reaction sites wherein said plurality of reaction sites comprise a plurality of reactants bound thereto for detecting said analyte, and c) a system of fluidic channels to allow said biological fluid and said plurality of reactants to flow in said apparatus, wherein at least one channel located between said plurality of reaction sites comprises an optical barrier to reduce the amount of optical cross-talk between said plurality of said reaction sites during detection of said analyte.
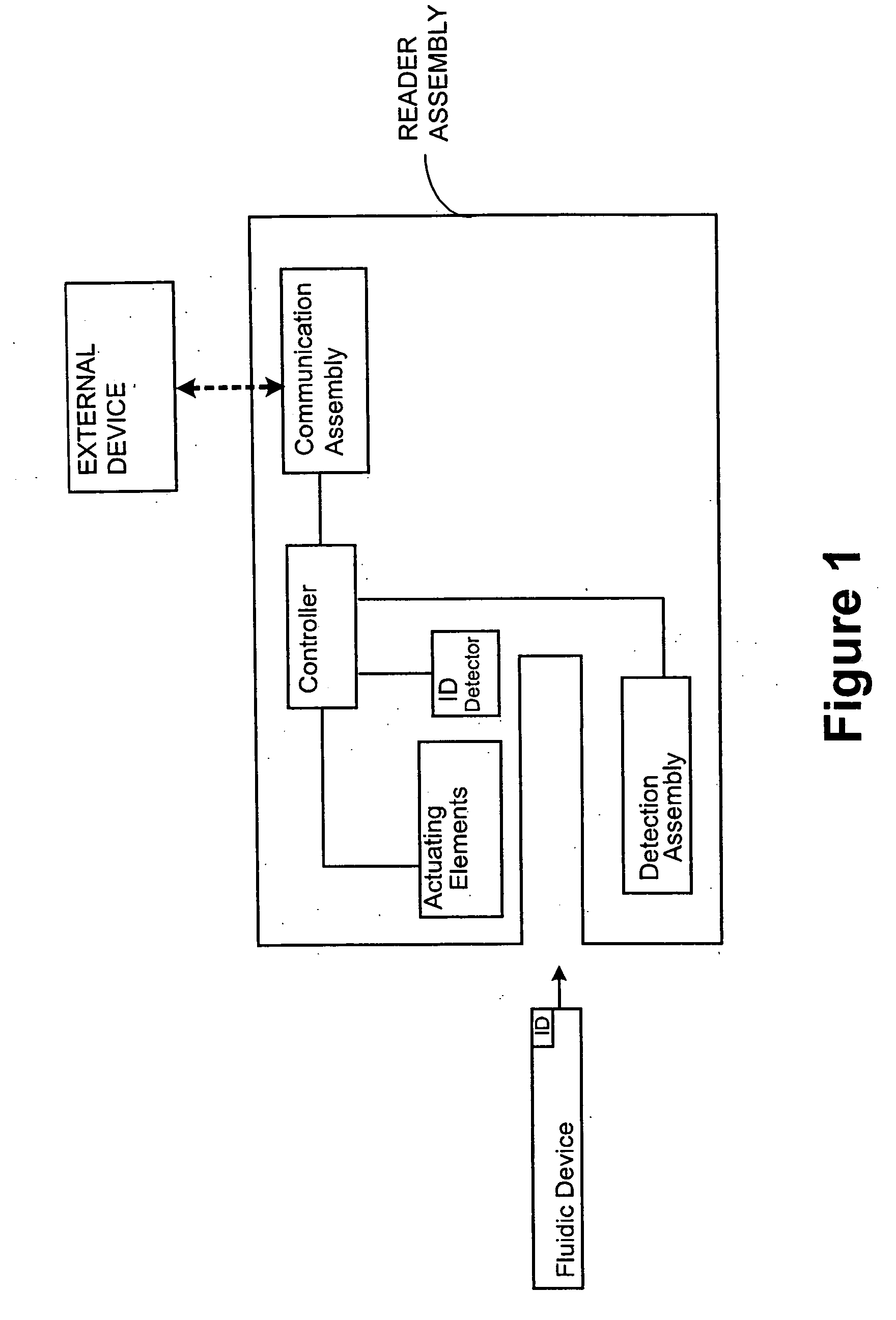
[0028] Where desired, the system may further comprise a reader assembly and a communication assembly. The sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com