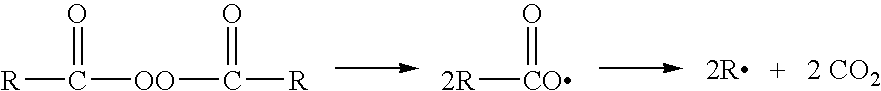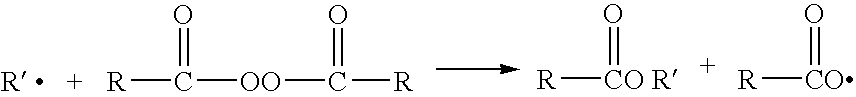Stable organic peroxide compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0050] A solution of benzoyl peroxide (“BPO”) was formulated in the following manner to deliver 8% benzoyl peroxide in the finished product.
IngredientAmountBenzoyl Peroxide 75% wet with water10.67 to carry in 8parts dry BPOBenzoyl benzoate40.00%
[0051] Benzoyl peroxide was dissolved into the benzyl benzoate. The resulting solution / dispersion was then added to the following materials.
IngredientAmountEthoxydiglycol10.00 parts Dimethyl Isosorbide41.1 partsButylated Hydroxytoluene (BHT) (antioxidant)0.40 partsVitamin E Acetate (antioxidant)0.50 parts
[0052] The above formulation results in a clear solution that has pharmaceutical properties.
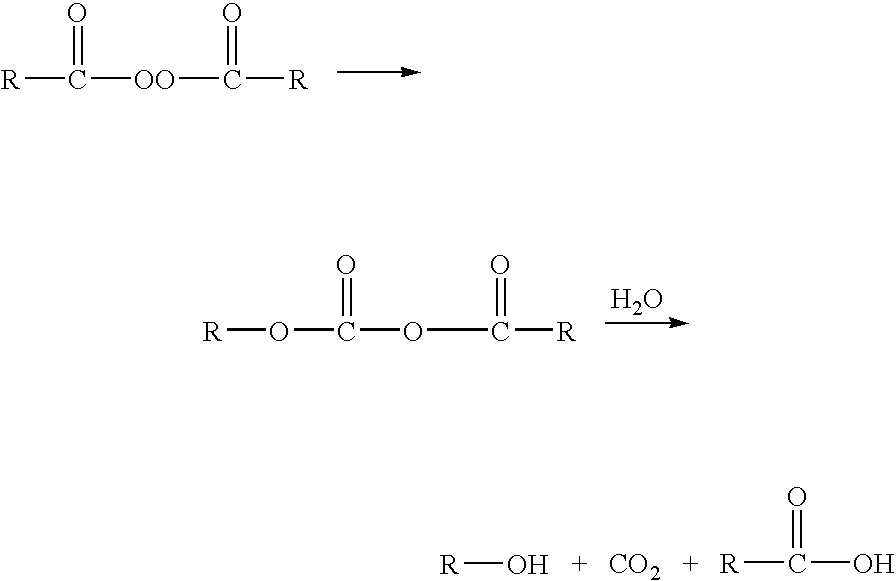
[0053] The thermal decomposition of benzoyl peroxide results in the generation of carbon dioxide gas as well as free radicals. The amount of carbon dioxide gas given off can be used as a relative measurement of the stability of any two compositions in relationship to each other.
[0054] The formula of Example 1 was placed on stability at elevated t...
example 2
[0057] A toner composition shown below was tested using the procedure described in Example 1.
IngredientAmountBenzoyl Peroxide 75% wet with water3.33% to carry in2.5% BPO dryEthoxydiglycol25.00% Benzyl benzoate42.47% Dimethyl isosorbide21.6%Benzoic acid5.00%Salicylic acid2.00%Vitamin E Acetate 0.2%Butylated hydroxyl toluene 0.4%
[0058] The test formula above was placed on stability at elevated temperatures of 40° C. and 30° C. versus a control formulation (the same formula above without the two antioxidants). The samples were placed in glass bottles with eye droppers and checked for the amount of gas that was generated. After a month at 40° C. the control samples (the same formula above without the two antioxidants) had filled up into the rubber bulb and pressure was evident via bulb expansion. In the case of the test formula, the droppers were empty and liquid had not moved into bulb. For the 30° C. samples the control had completely filled the dropper and was present in the bulb. ...
example 3
[0059] Another formulation in accordance with the present disclosure is as follows:
IngredientAmountBenzoyl Peroxide 6.25%Benzoyl benzoate42.45%Dimethyl isosorbide40.00%Vitamin E Acetate 0.5%BHT 0.8%Ethoxy diglycol 10.0%fumed silica0-10%
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com