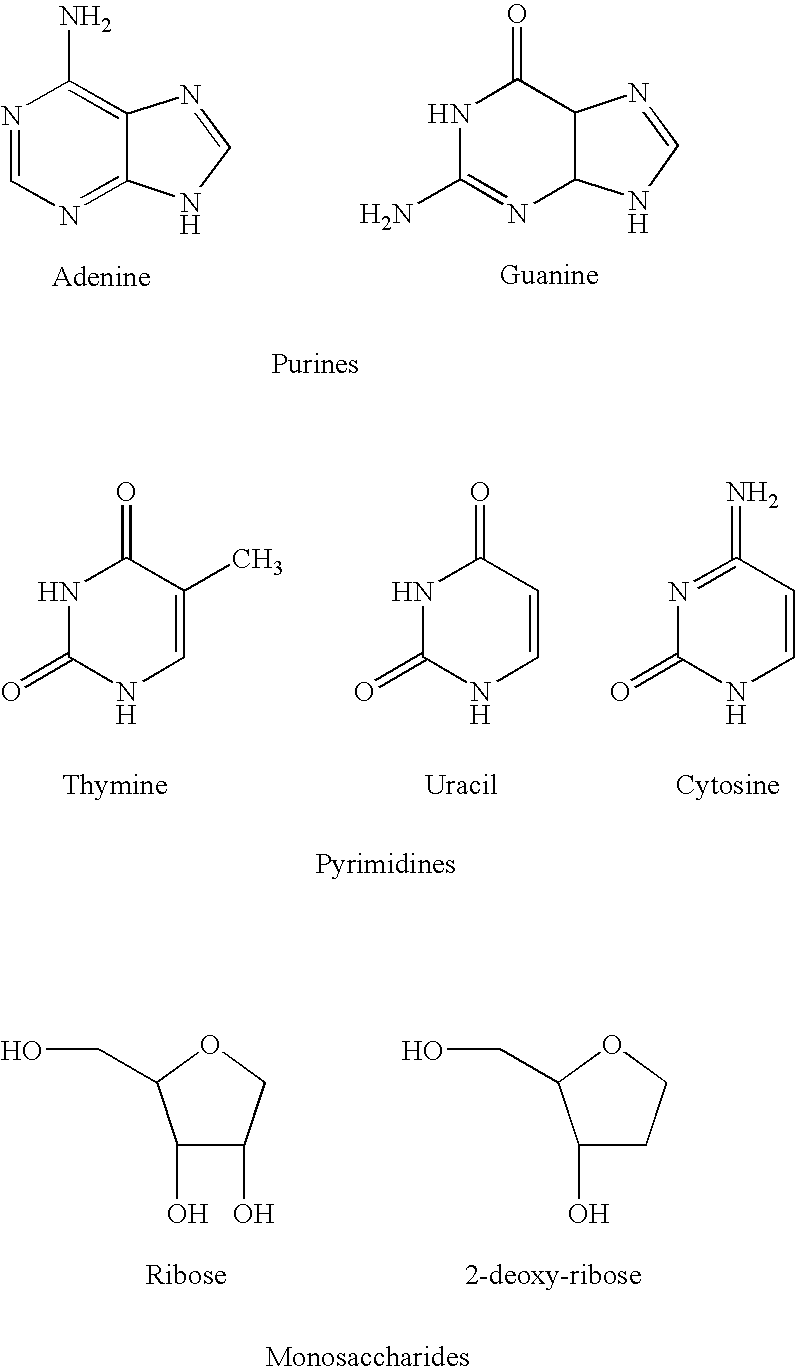
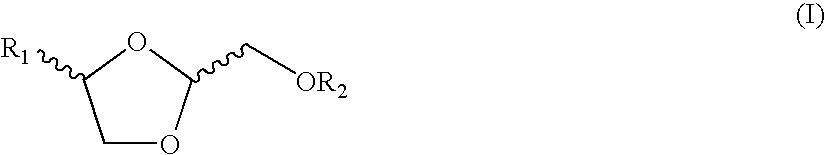Chemical compounds
a technology of fatty acid derivatives and compounds, applied in the field of chemical compounds, can solve the problems of inferior specificity for treatment of actual disease, low activity, and nocleoside analogues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] The breast cancer cell line MaTu was seeded, 5×103 cells per well, in 96-well-plates. The medium was RMPI 1640 with 2 mM Glutamine and 10% Foetal Bovine Serum. 24 hours later the test compounds were added in 6 concentrations. The cells were incubated for 4 days. The MTT solution was added to each well and incubated for 4 hours. The samples were read by an ELISA reader at 540 nm. The IC50 values were determined from growth curves. The test compounds were troxacitabine elaidic amid, troxacitabine petroselaidoate, troxacitabine-petroselaidic amide and troxacitabine γ-linolenoate. Surprisingly the activity of troxacitabine petroselaidoate and petroselaidic amide was 20 fold more active than troxacitabine elaidic amid.
IC50 μM ±CompoundStandard Deviationtroxacitabine elaidic-amide3.58 ± 2.56troxacitabine petroselaidoate0.18 ± 0.12troxacitabine-petroselaidic amide0.15 ± 0.07troxacitabine γ-linolenoate0.83 ± 0.59
example 2
[0023] The human breast carcinoma cancer cell line MaTu and the adriamycin resistant cell line MaTu / ADR were seeded, 5×103 cells per well, in 96-well-plates. The medium was RMPI 1640 with 2 mM Glutamine and 10% Foetal Bovine Serum. 24 hours later the test compounds were added in a final volume of 20 μl to the cells, in six different concentrations. The cells were incubated for 4 days. MTT solution was added to each well and incubated for 4 hours. The samples were read by an ELISA reader at 540 nm. The IC50 values were determined from growth curves. The resistance factor is the IC50 in MaTu / Adr vs. IC50 in MaTu. The test compounds were troxacitabine-elaidic amide and troxacitabine-petroselinic amide. Surprisingly we found the troxacitabine-petroselinic amide to be independent of the adriamycin resistance, with a resistance factor of 1.0, compared to 5.8 for troxacitabine-elaidic amide.
Resistance factor =Compound(IC50 MaTu / Adr) / (IC50 MaTu)troxacitabine- elaidic amide5.8troxacitabine...
example 3
Troxacitabine N4-elaidic Acid Amide (Comparative Compound)
[0024] Troxacitabine (150 mg, 0.70 mmol), TEA (0.1 ml, 0.74 mmol) and DMAP (90 mg, 0.74 mmol) in dry DCM / DMF (5 ml / 2 ml) was added elaidoyl chloride in DCM (5 ml). The acid chloride was prepared from elaidic acid (209 mg, 0.74 mmol), oxalyl chloride (0.4 ml, 2.96 mmol) and DMF (catalytic amount) in toluene (10 ml) by stirring at ambient temperature for 2 h and then evaporated to dryness. After stirring for 22 h at room temperature a saturated aqueous solution of NH4Cl was added and the phases separated. The aqueous phase was extracted with DCM (3×), and the combined organic extracts were washed with saturated brine, dried (Na2SO4), filtered and evaporated in vacuuo. The product was purified by flash chromatography on silica gel eluting with MeOH / DCM (25:975) followed by MeOH / DCM (5:95) to give 208 mg (62%) of the desired product as colourless crystals.
[0025]1H-NMR (200 MHz; CDCl3); δ 8.51 (1H, d), 7.43 (1H, d), 6.21 (1H, m)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com