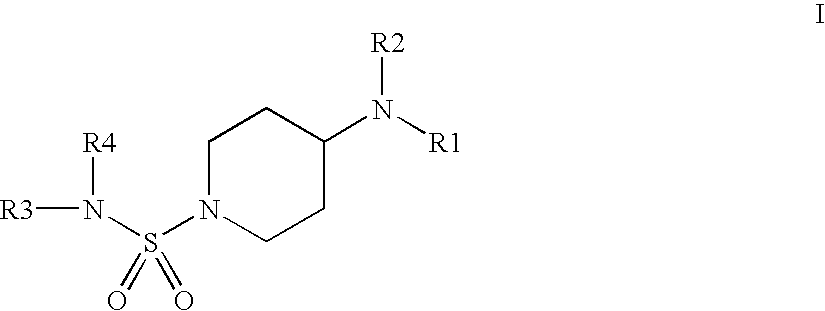
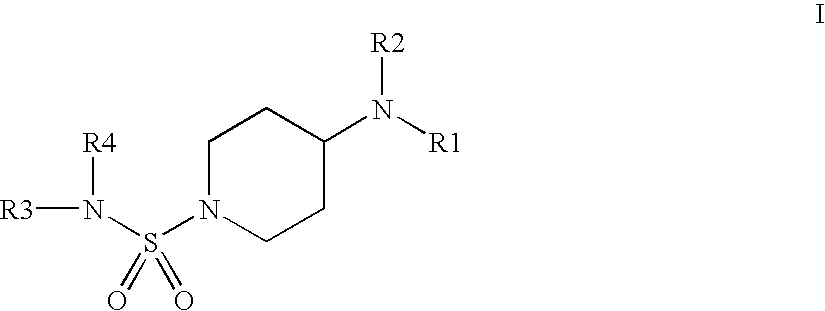
N-Sulfamoyl-piperidineamides for the treatment or inhibition of obesity and related conditions
a technology of n-sulfamoylpiperidineamide and n-sulfamoylpiperidineamide, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of complex biological mechanisms at the molecular level between insulin resistance and metabolic risk factors, inability to maintain a proper level of glucose, and no well-accepted criteria for diagnosing metabolic syndrom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0113] Urea-analogs (R1═CH2C6H5, R2═CO—NH—C6H5; boc=tert.-butyloxycarbonyl) [0114] 2.1 2.6 g sodium acetate, 5.0 g tert.-butyl-4-aminopiperidine-1-carboxylate, 2.0 ml acetic acid and 2.1 ml benzaldehyde were combined in 200 ml THF and stirred for 4 hrs. at room temperature. After addition of 8.8 g trisacetoxy sodiumborohydride the mixture was stirred for 20 hrs. Then solvent was removed under reduced pressure and the residue was dissolved in a mixture of methyl-tert.-butylether and water. The aqueous layer was made alkaline with NaOH and extracted twice with methyl-tert.-butylether. The combined organic layers were washed three times with 30 ml 0.1N HCl and 5 times with 50 ml 0.1N HCl. Then the aqueous layers were combined and made basic with NaOH, followed by two extractions with methyl-tert.-butylether. The organic layers were washed with water and a saturated solution of NaCl in water, dried over sodium sulfate and then evaporated in vacuum. 4.9 g tert.-butyl 4-(benzylamino)pipe...
example 3
[0121] Substituted amides (R1=CH3; R2=C6H11; boc=tert.-butyloxycarbonyl) [0122] 3.1 2.0 g tert.-butyl 4-oxopiperidine-1-carboxylate, 1.23 g sodium acetate, 0.98 ml acetic acid and 1.56 ml N-methylcyclohexanamine were dissolved in 100 ml THF and stirred for one hour at room temperature. Then 4.25 g trisacetoxy sodiumborohydride were added and the reaction mixture was stirred for 18 hours at room temperature. The reaction mixture was concentrated under reduced pressure and the residue was taken up in a mixture of water and methyl-tert.-butylether. The aqueous layer was made alkaline and extracted twice with methyl-tert.-butylether. Finally, the organic layer was washed 2 times with 0.1N HCl, the aqueous layers were combined and made alkaline (pH 10) by addition of NaOH solution. After extraction (2 times) with methyl-tert.-butylether the organic layer was dried over sodium sulfate and evaporated in vacuum. 1.3 g of oily tert-butyl 4-[cyclohexyl-(methyl)amino]piperidine-1-carboxylate ...
example 4
[0130] Substituted amides (R1═H; R2═C6H11; boc=tert.-butyloxycarbonyl) [0131] 4.1 1.23 g sodium acetate, 2.4 g tert.-butyl-4-aminopiperidine-1-carboxylate, 1.0 ml acetic acid and 1.0 g cyclohexanone were combined in 150 ml THF and stirred for 3 hrs. at room temperature. Then 4.25 g trisacetoxy sodiumborohydride were added and the reaction mixture was stirred for 16 hrs. at room temperature. After concentrating the reaction mixture under reduced pressure the residue was taken up in a mixture of methyl-tert.-butylether and water, which was made alkaline with sodium carbonate to pH 9. The organic layer washed 4 times with 0,1N HCl. Then the aqueous layer was made alkaline with NaOH and extracted twice with methyl-tert.-butylether. The combined organic layers were washed with water and with a saturated solution of NaCl in water, dried over sodium sulfate and then evaporated under reduced pressure, yielding 2.4 g of oily tert-butyl 4-(cyclohexylamino)piperidine-1-carboxylate.
[0132]1H N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com