Reducing imunogenicities of immunoglobulins by framework-patching
a framework-patching and immunoglobulin technology, applied in the field of new antibodies, can solve the problems of difficult identification of the proper framework amino acids to be replaced, the magic bullet concept took more than 25 years to realize, and the loss of affinity for the antigen, etc., to achieve the effect of reducing or eliminating immunogenicities and being flexibl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
FR-Patched Anti-CD22 Antibody
Design of Genes for FR-Patched Anti-CD22 Light and Heavy Chain
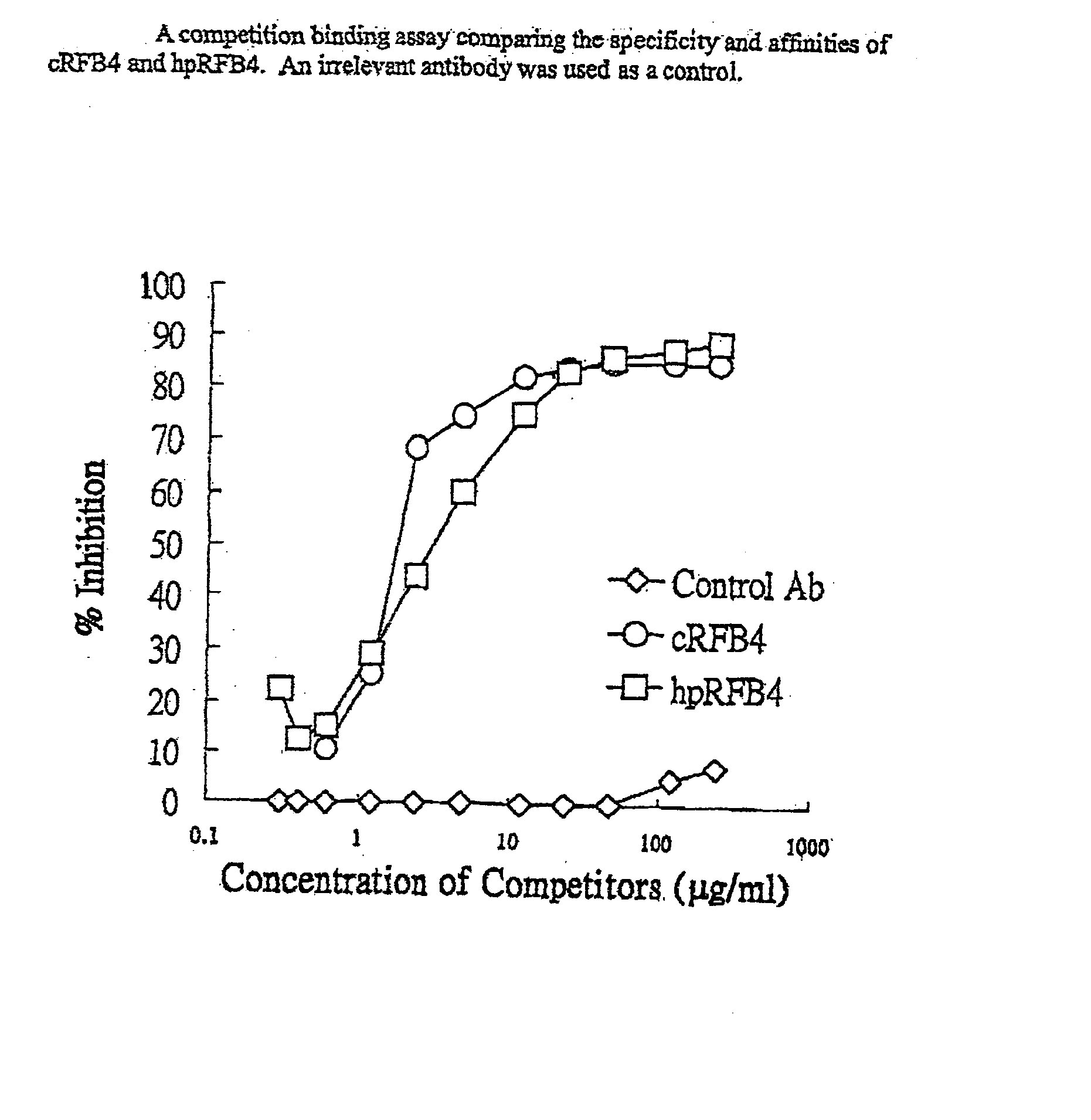
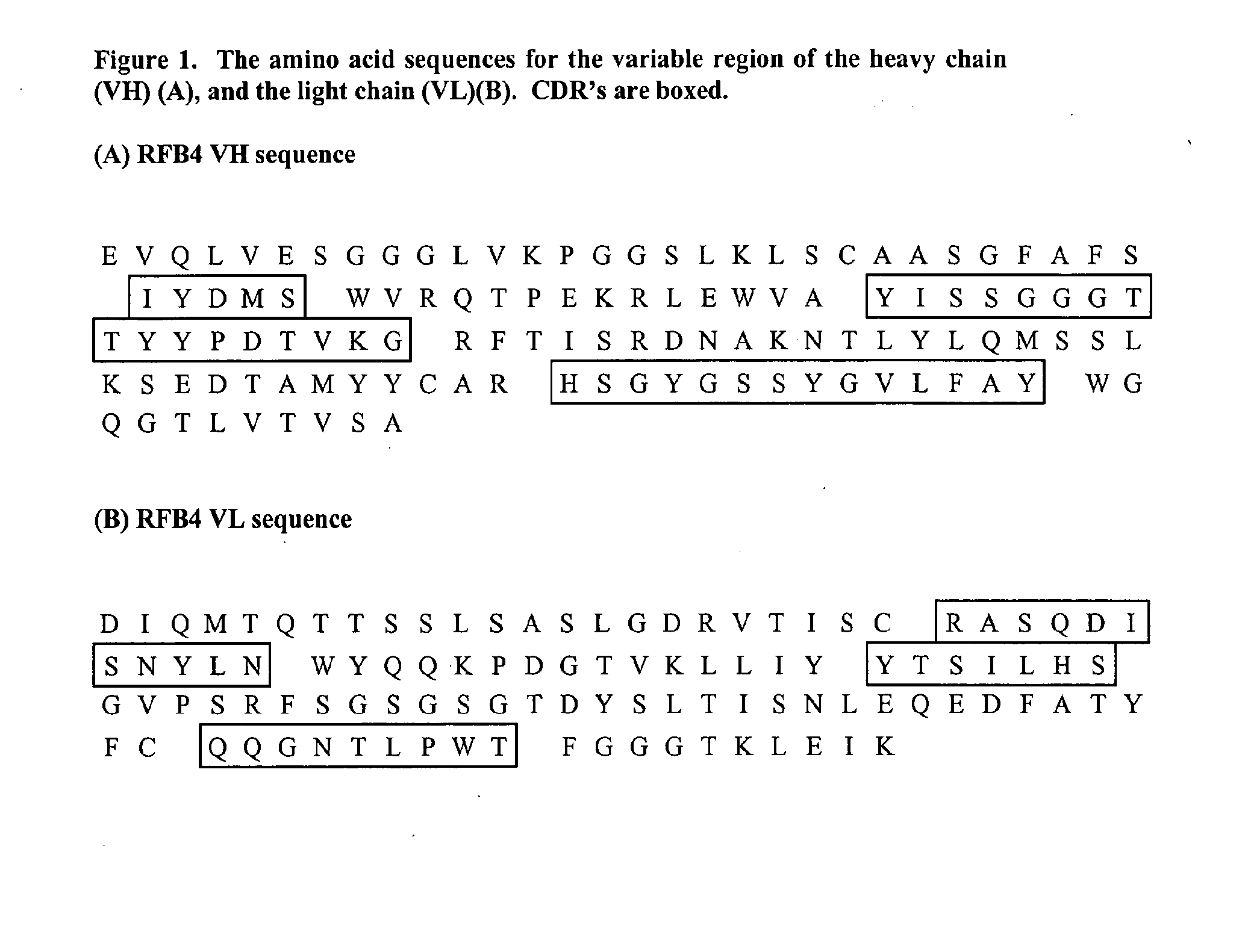
[0072] The heavy and light chain sequence of a murine anti-CD22 antibody, RFB4 (Li et al., Cell Immunol. 118:85, 1989; Mansfield et al., Blood 90:2020-2026, 1997) is used as an example to illustrate the approach of using FR-patching to reduce or eliminate immunogenicity of the re-engineered antibody. The sequences of the heavy (a) and light chain (b) variable region for the murine antibody are shown in FIG. 1.
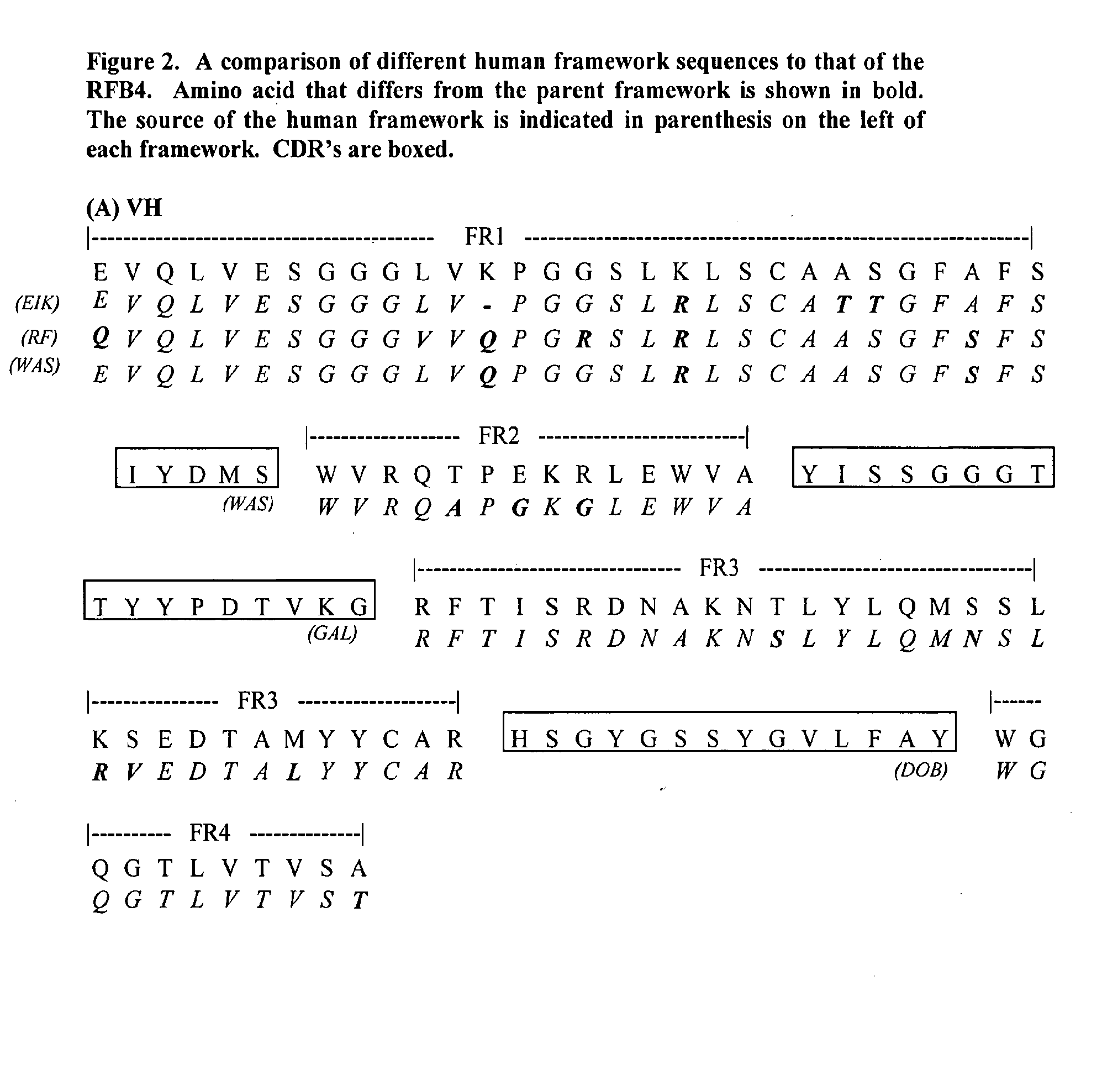
[0073] Patching of the individual FRs for the heavy chain variable region for RFB4 was done as follows: [0074] a. FR1: the FR1 sequence of the murine VH was compared with the FR1 sequences of human VH from the Kabat's database (Kabat et al., op. cit.). Although human FR1 of the highest sequence homology is preferred, particular emphasis on the sequence closest to the CDR1 was taken. There are three FR1 sequences that are of high homology to the murine FR1. They are, namely, EIK, RF-SJ...
example 2
FR-Patched Anti-CD20 Antibody
Design of Genes for FR-Patched Anti-CD20 Light and Heavy Chain.
[0110] The heavy and light chain sequence of a murine anti-CD20 antibody, 1F5 (Ref.) is used as an example to illustrate the approach of using FR-patching to reduce or eliminate immunogenicity of the re-engineered antibody. The sequences of the heavy and light chain variable region for the murine antibody are shown in FIG. (7).
[0111] In designing the amino acid sequence of the FR-patched immunoglobulin for 1F5, the same set of rules as described previously applies. However, there are always situations when no appropriate FR's fulfill all the above-mentioned requirements. The FR-patching approach offers a great degree of flexibility allowing the introduction of murine residues in the problematic FR's, or alternatively, inclusion of the original murine FR's without modifications. The resultant FR-patched antibody will presumably have significantly reduced immunogenicity compared to a murine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com