Apparatus For (Meth) Acrylic Acid Production And Process For Producing (Meth) Acrylic Acid
a technology of acrylic acid and apparatus, which is applied in the preparation of carboxylic compounds, indirect heat exchangers, lighting and heating apparatus, etc., can solve the problems of increasing pressure in the reactor, clogging of the heat exchanger, and developing difficulties in continuing a usual operation, etc., to achieve stable and continuous operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
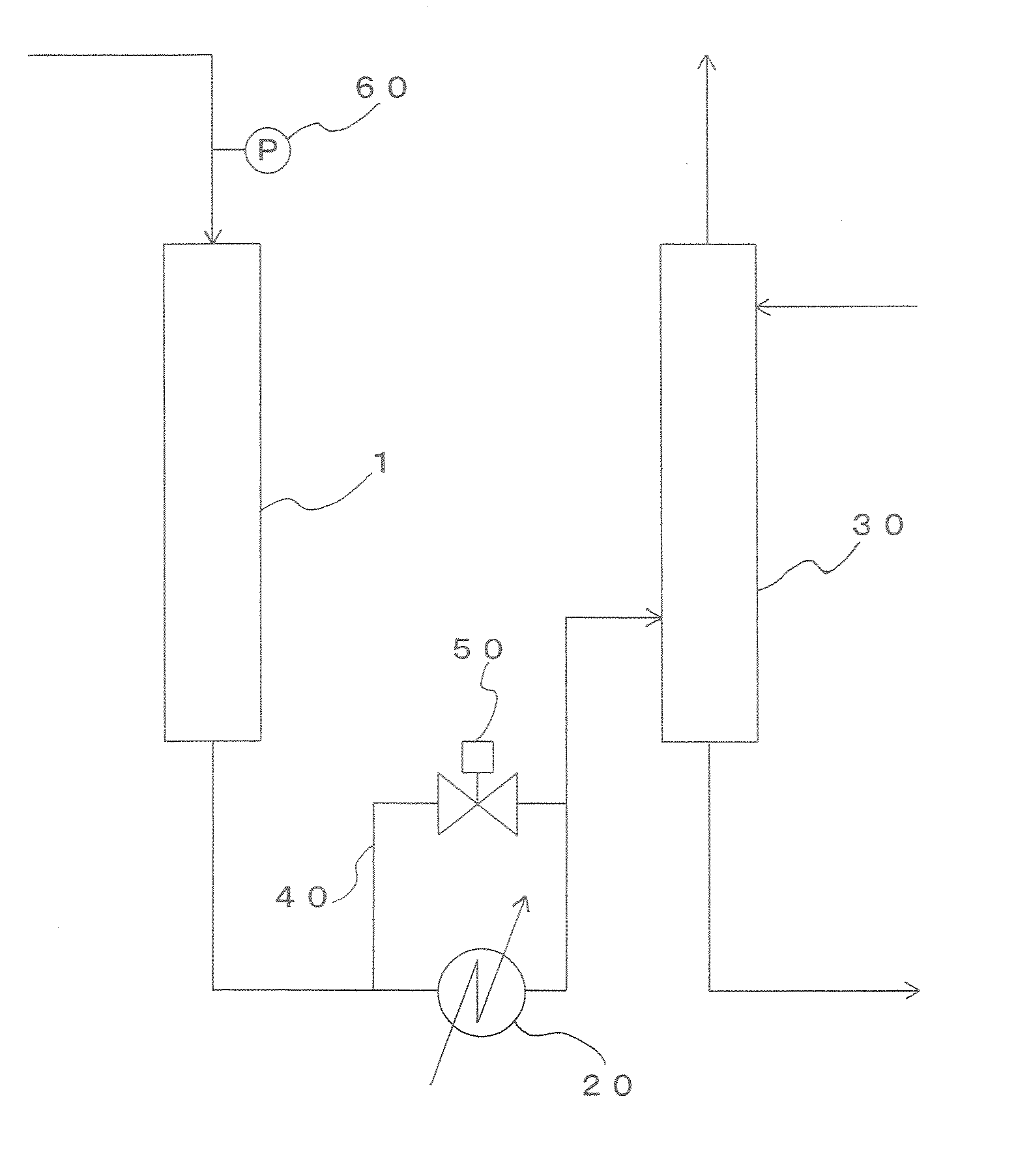
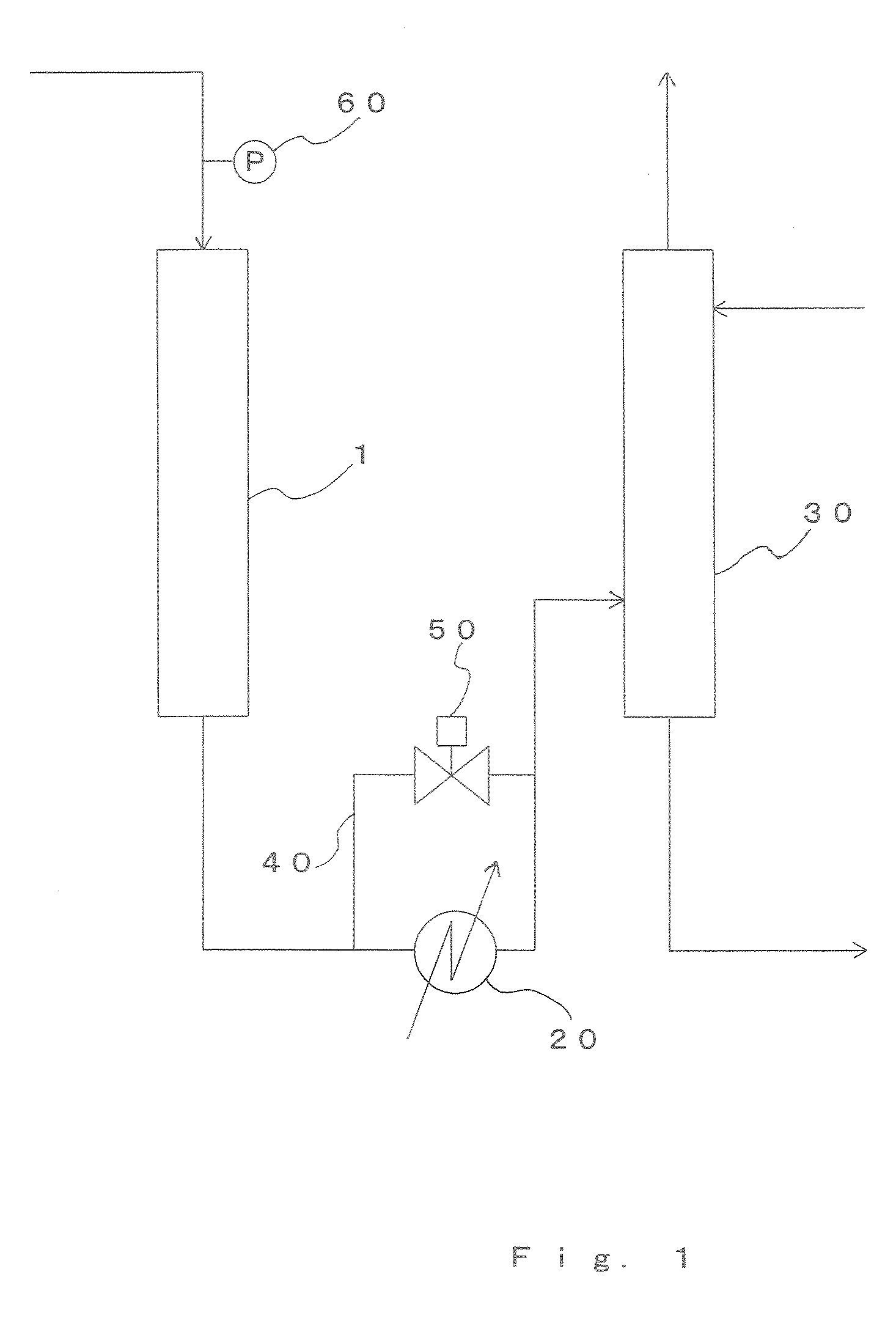
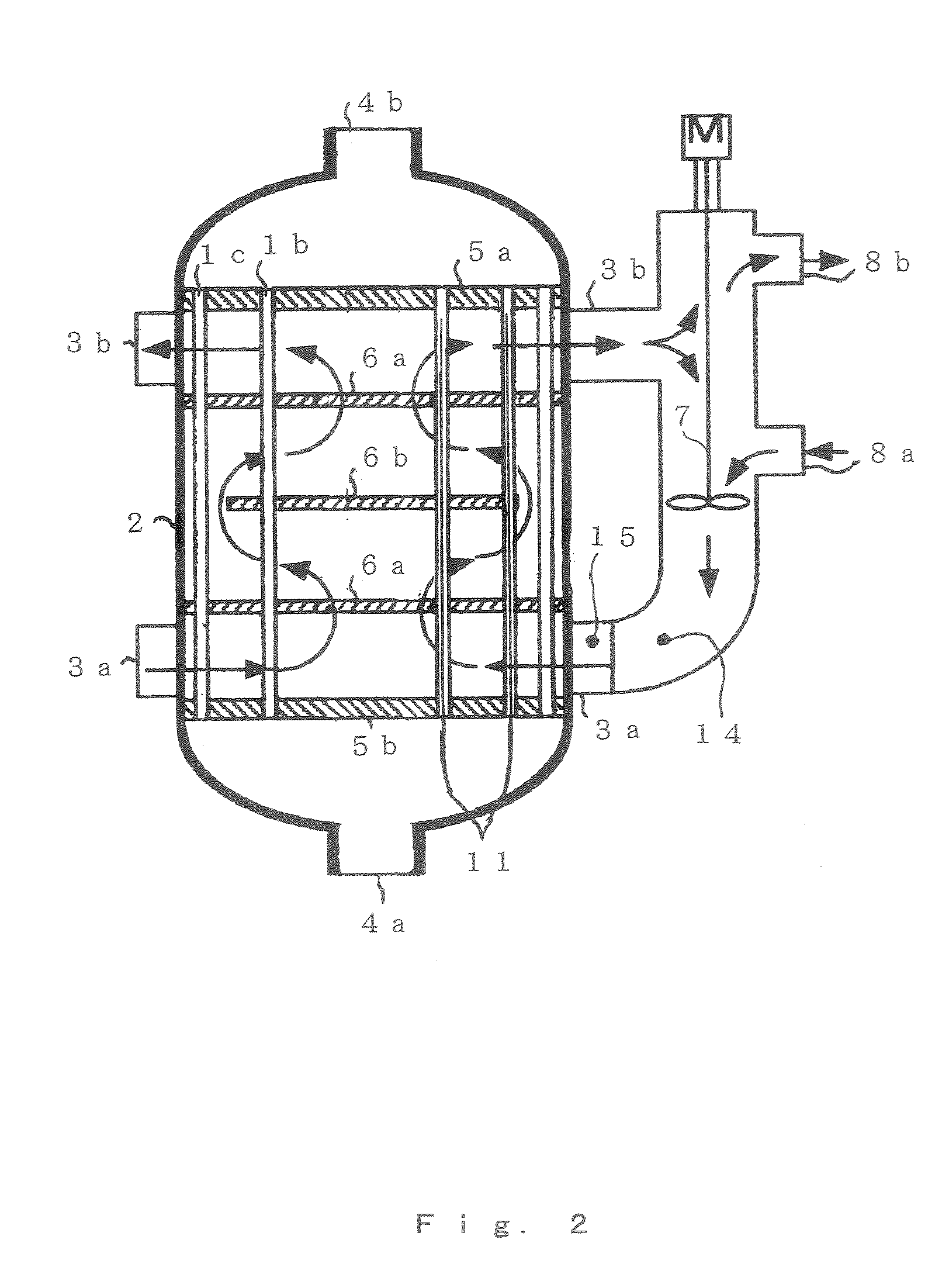
[0113] Acrylic acid was produced through a vapor-phase catalytic oxidation reaction of propylene using the production apparatus shown in FIG. 1. The multitube reactor shown in FIG. 3 was used as the reactor 1.
[0114] A catalyst composed of a mixed oxide having an atomic ratio of Mo:Bi:Co:Ni:Fe:Na:Mg:B:K:Si=12:5:2:3:0.4:0.1:0.4:0.2:0.08:24 disclosed in JP 06-013096 B as an oxidation catalyst for oxidizing propylene to produce mainly acrolein was packed in reaction tubes of a first stage (hereinafter referred to as “first reactor”) of the multitube reactor.
[0115] On the other hand, a catalyst composed of a mixed oxide having an atomic ratio of Mo:V:Nb:Sb:Sn:Ni:Cu:Si=35:7:3:100:3:43:9:80 disclosed in JP 11-035519 A as a catalyst for oxidizing acrolein to produce acrylic acid was packed in reaction tubes of a second stage (hereinafter, referred to as “second reactor”) of the multitube reactor.
[0116] Liquefied propylene was passed through an evaporator and was supplied to the reactor 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com