Methods for regulating T cell subsets by modulating transcription factor activity
a transcription factor and activity-modulating technology, applied in the direction of peptides, drug compositions, immunological disorders, etc., can solve the problems of unwanted side effects of cytokines or antibodies, and achieve the effects of stimulating expression or activity, modulating the activity of transcription factors, and enhancing the expression or activity of transcription facto
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cytokine Specificity is Due to a Positive Transacting Factor and not to a Repressor
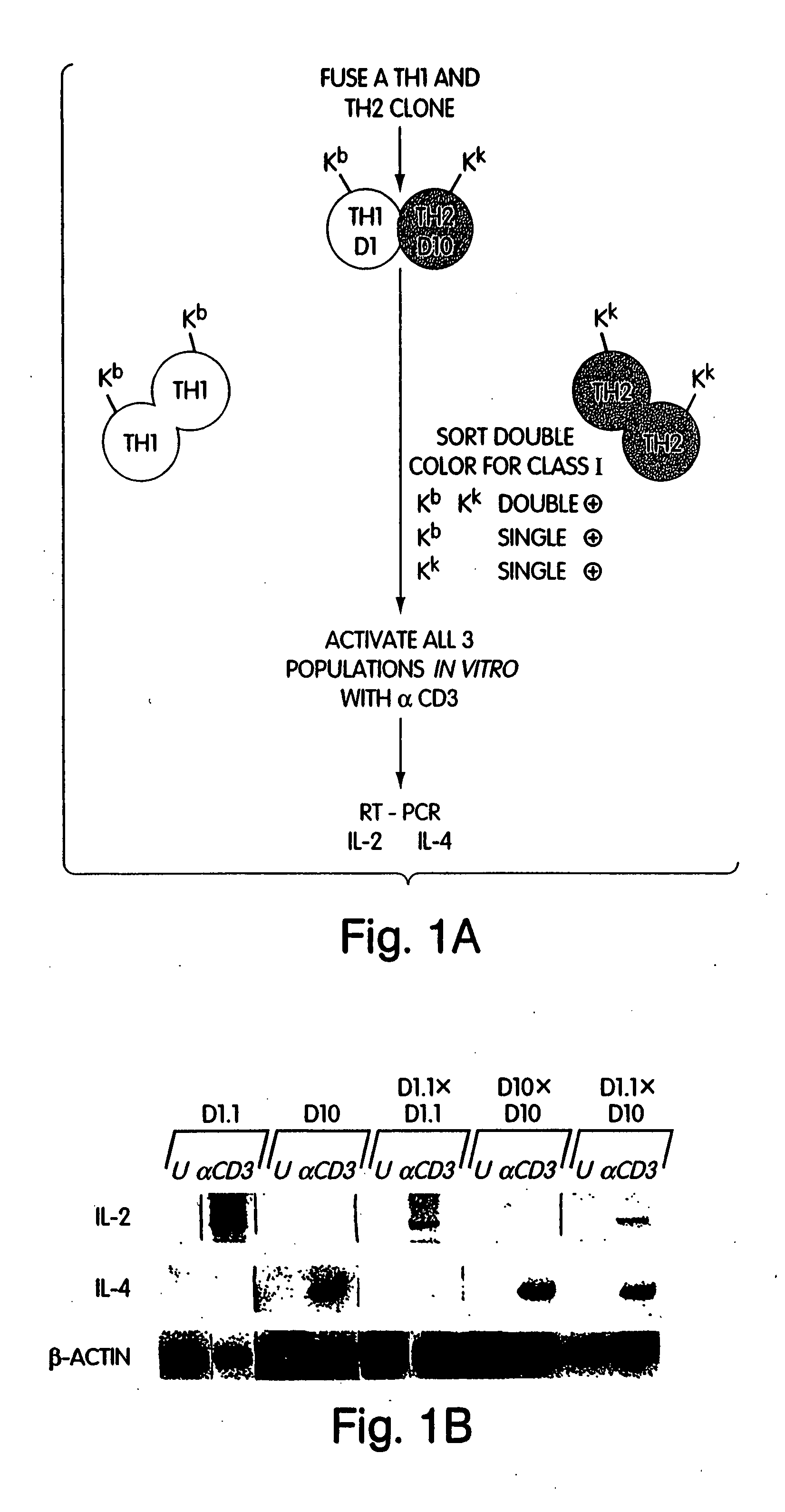
[0205] Tissue specificity can be achieved through the action of repressor or silencer proteins. Thus it was possible that the IL-2 and IL-4 genes were actively repressed in Th2 and Th1 cells respectively. To test for the existence of repressor proteins, somatic cell fusions were performed between a Th1(D1.1) and a Th2 (D 10) clone of differing MHC Class I haplotypes. The Th1 clone D1.1 (Kd) and the Th2 clone D10 (Kk) were fused according to the “suspension cell fusion” procedure (Lane, R. D. et al. (1986) Methods Enzymol. 121:183-192). After fusion, the cells were allowed to recover for 8 hours and then double-stained using PE-conjugated anti-Kk and FITC-conjugated anti-Kd antibodies (Pharmingen, La Jolla, Calif.). Cells were then sorted on the basis of size to distinguish unfused cells from hetero and homokaryons and by fluorescence to identify single-positive and double-positive cells. As indicated...
example 2
Isolation of a Th2-Specific c-maf Gene from a cDNA Library Prepared from an Anti-CD3 Activated Th2 Clone
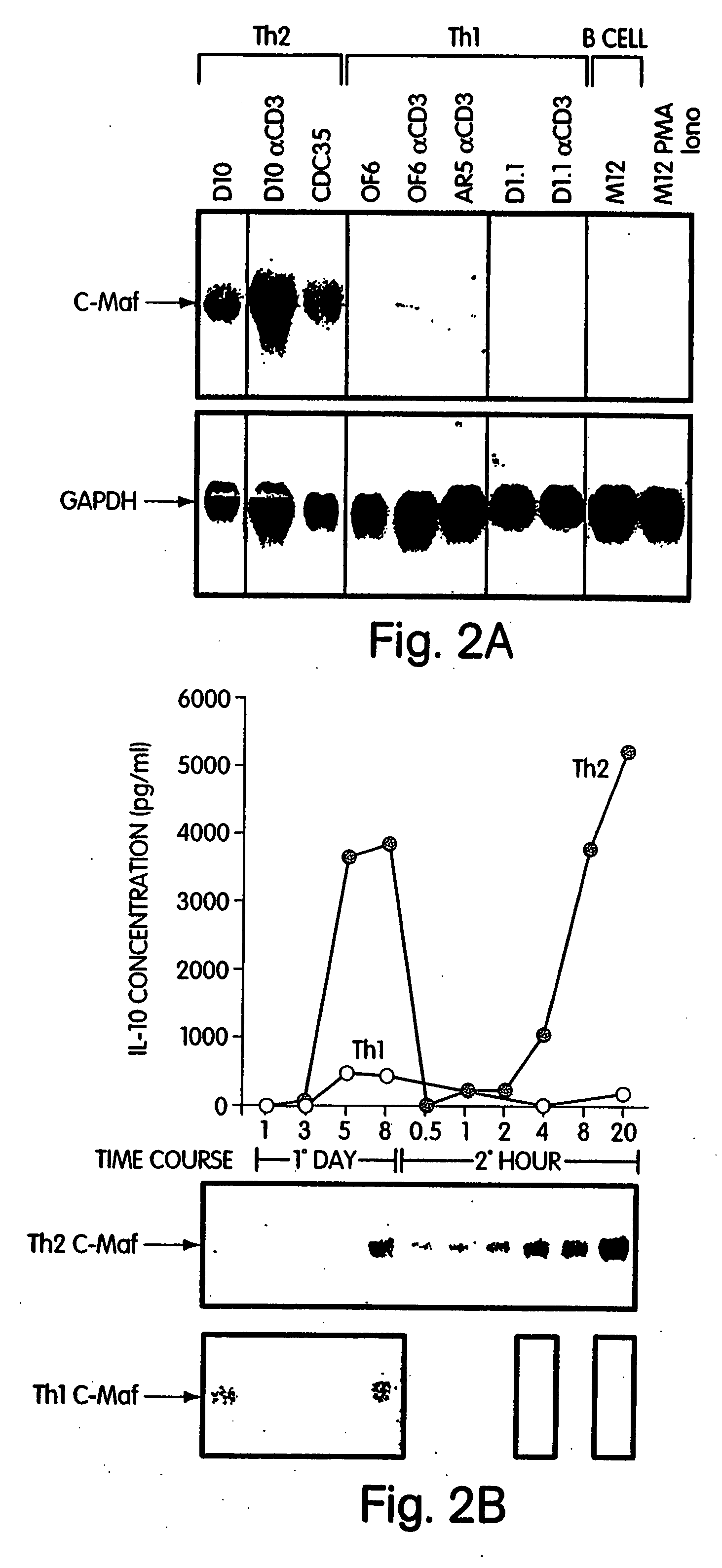
[0208] In the course of screening a cDNA library prepared from an anti-CD3 activated Th2 clone, D10, for NF-AT-interacting proteins by the yeast two-hybrid system (for descriptions of this system, see e.g., Field U.S. Pat. No. 5,283,173; Zervos et al. (1993) Cell 72:223-232; Madura et al. (1993) J. Biol. Chem. 268:12046-12054; Bartel et al. (1993) Biotechniques 14:920-924; and Iwabuchi et al. (1993) Oncogene 8:1693-1696), multiple cDNAs were isolated, all of which were extremely weak interactors. All cDNAs obtained in this screen were next evaluated for Th-specific expression by Northern blot analysis using a panel of Th1 and Th2 clones. One such cDNA, which was repeatedly isolated (60 of 140) detected transcripts only in RNA prepared from Th2 clones (D10, CDC35) and not from either Th1 clones (AR5, OS6, D1) or from a B cell lymphoma, M12, as illustrated in the Northern blot anal...
example 3
Ectopic Expression of c-Maf in Th1 and B Cells Results in Activation of the IL-4 Promoter
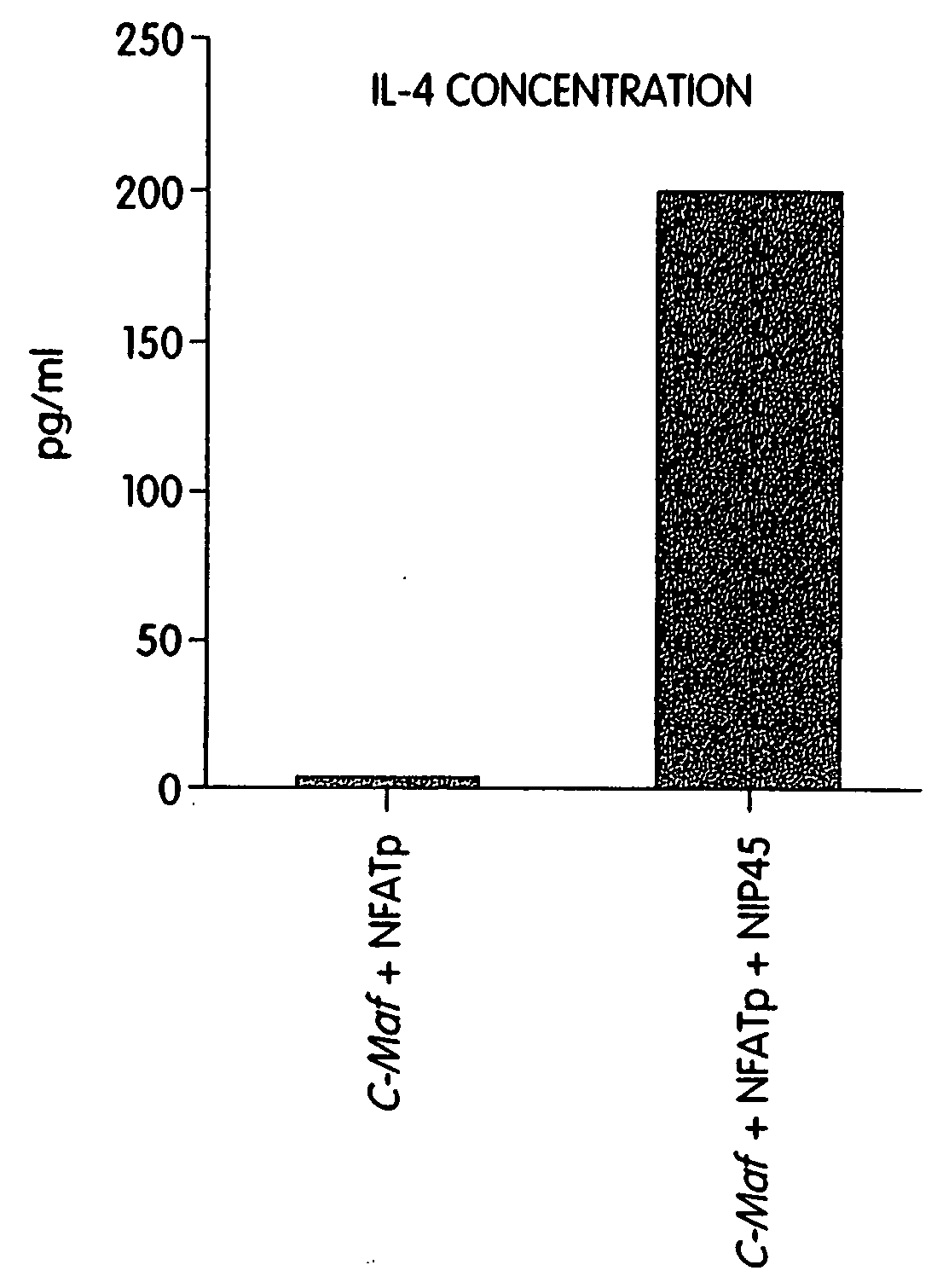
[0213] The identification of the isolated cDNA described in Example 2 as a member of the AP-1 / CREB / ATF gene family, together with its selective expression in Th2 cells raised the possibility that c-Maf controlled the tissue-specific transcription of the IL-4 gene. Additionally, the presence of transcripts encoding c-maf correlated well with IL-4 expression in Th2 cells and in three of four transformed mast cell lines examined. To test whether c-Maf could transactivate the IL-4 promoter, cotransfection experiments were performed.
[0214] Th1 clones and the B lymphoma M12.4.C3 (M12) neither express c-maf nor transcribe the IL-4 gene. If c-Maf is the transcription factor critical for controlling IL-4 gene expression, then forced expression in these cells should permit IL-4 gene expression. To test this, the full-length (4.3 kb) c-maf cDNA clone was inserted into the SalI site of the pMex-NeoI mamma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com