Novel Antiangiogenic Peptide Agents and Their Therapeutic and Diagnostic Use
a technology of peptide agents and peptide sources, which is applied in the direction of peptide sources, angiogenin, growth hormones, etc., can solve the problems of pathological impairment, poor vascular development, rapid and excessive angiogenesis accompanies the growth of placenta and solid tumors,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antiangiogenic Activity of 16K N-Terminal Fragments of Human Growth Hormone, Human Placental Lactogen, Human Prolactin, and Human Growth Hormone Variant hGH-V
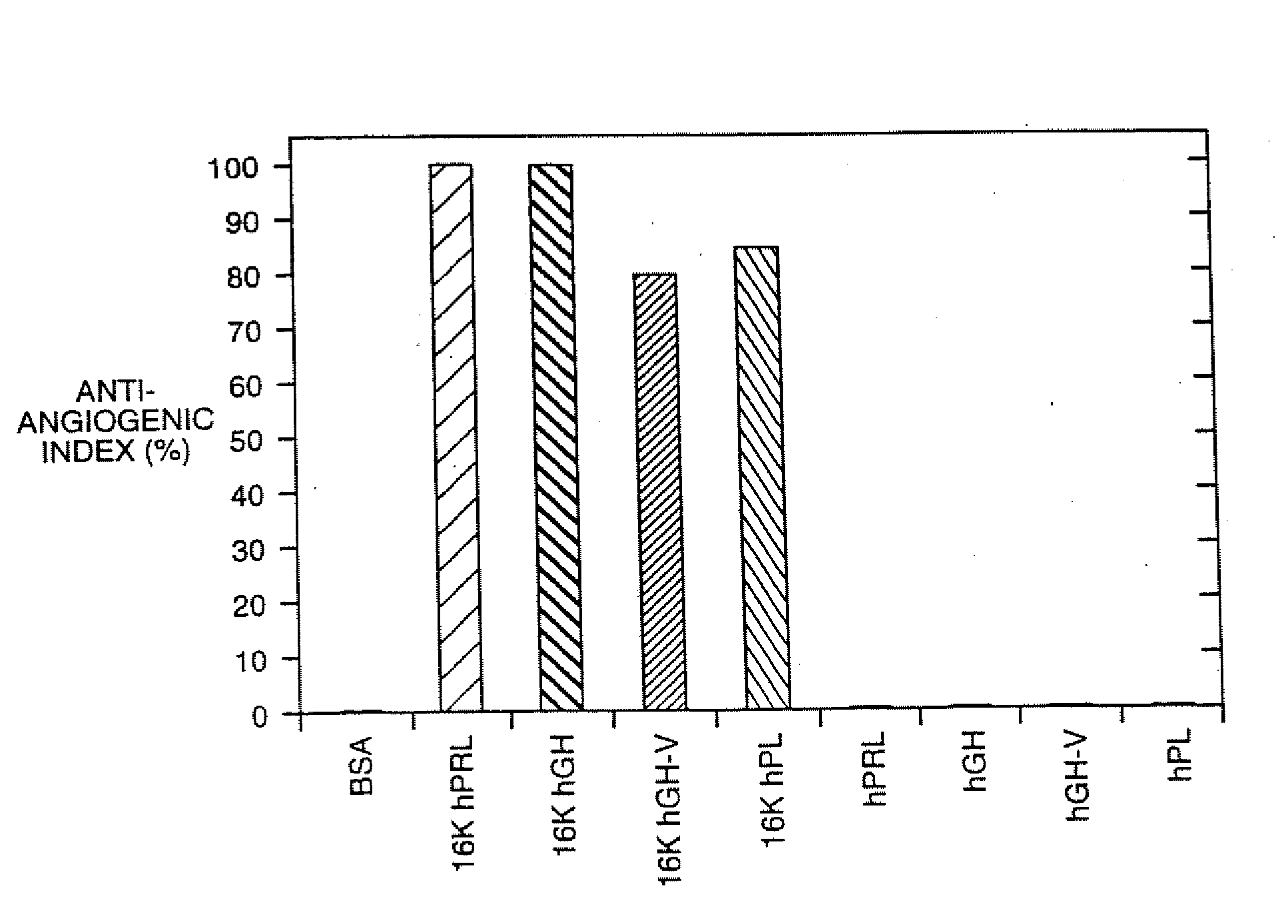
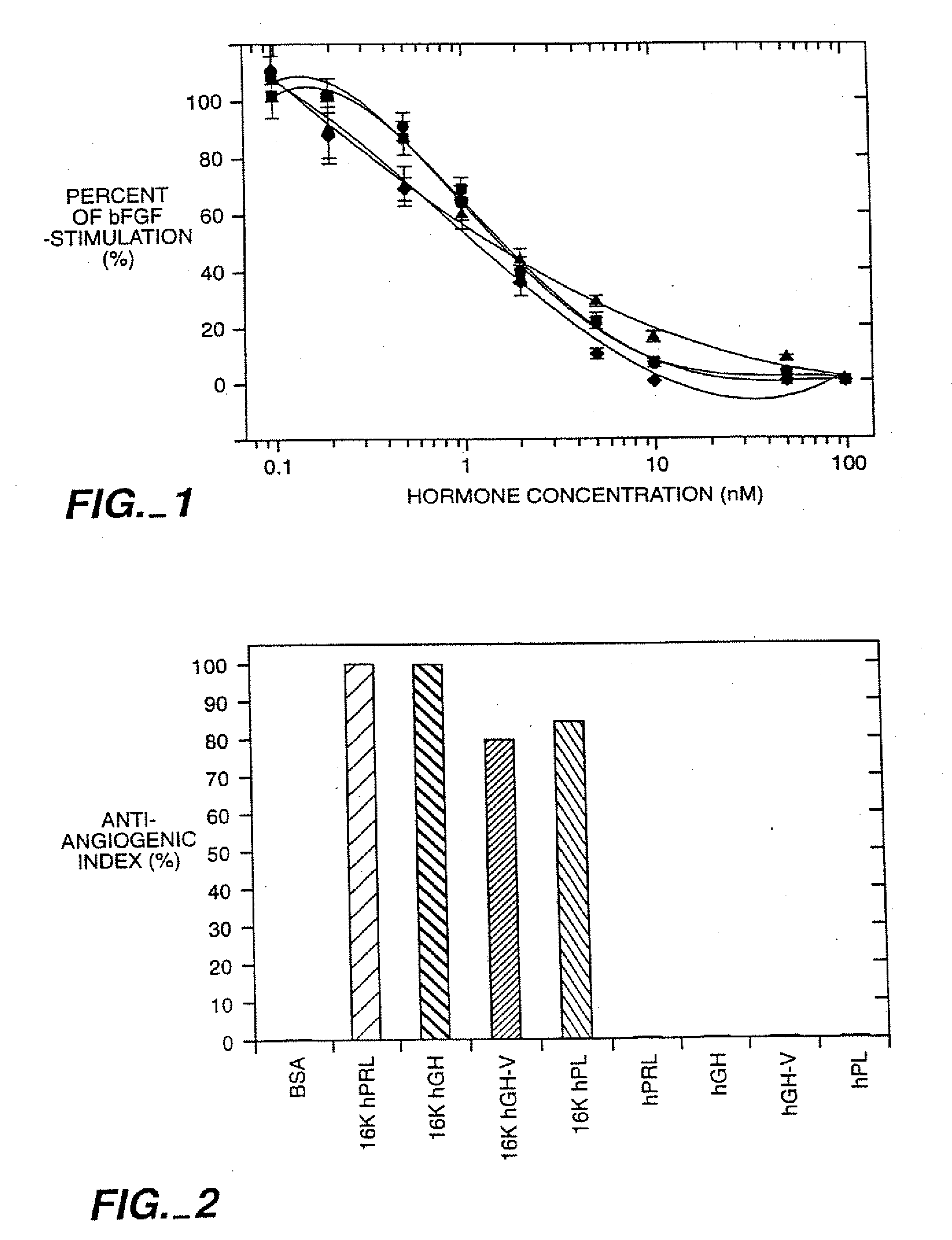
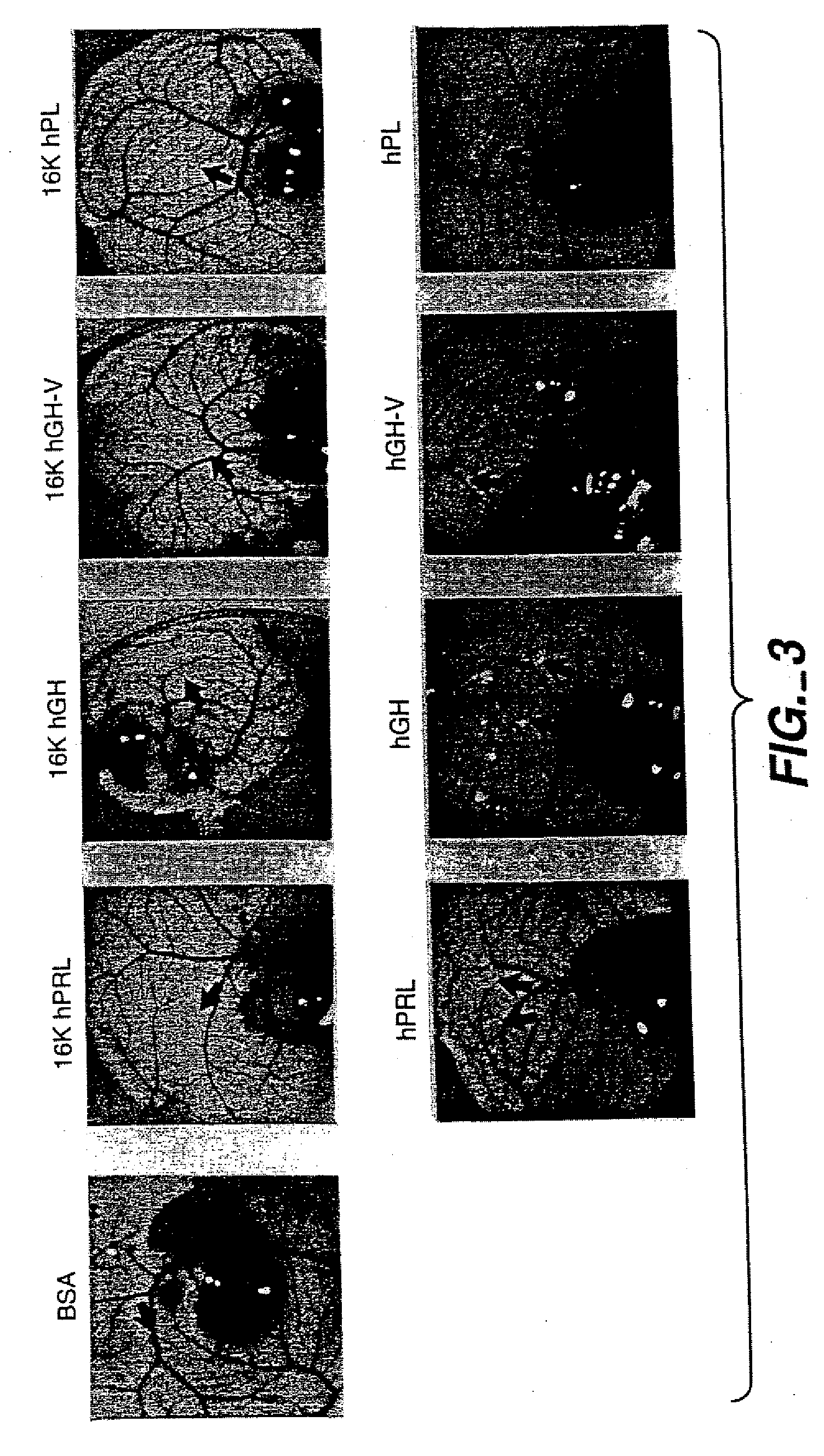
[0107] 16K N-terminal antiangiogenic agents were derived from full length human growth hormone, human growth hormone variant hGH-V, human placental lactogen and prolactin. These peptides were tested in vivo and in vitro for their binding and antiangiogenic activity in assays described in below. Results of these testings are illustrated in FIGS. 1-5.
[0108] A. In Vitro and In Vivo Angiogenic Inhibitory Activity
[0109] Biological antiangiogenic activity of the 16K N-terminal fragments was tested in bovine brain capillary endothelial cell assay, as well as in vivo chick chorioallantoic membrane assay. The in vitro and in vivo antiangiogenic inhibitory characteristics of the peptides of the invention were determined and are described in Sections 1 and 2, below.
[0110] 1. In Vitro Antiangiogenic Activity
[0111] In vitro, the peptid...
example 2
Endogenous 16K N-Terminal Fragments in the Placenta
[0151] The interaction of fetal and maternal cells at the placental-decidual interface in eutherian mammals has been the subject of intensive investigation during the last two decades. Variation among species is dramatic. The most complex interactions are observed in hemochorial placentation, e.g. in human and mouse, where fetal trophoblast cells are in direct contact with the maternal circulation.
[0152] The establishment of adequate placental vascularization is critical to the survival of the developing conceptus, and requires extensive proliferation and remodeling of the deciduil vascular tree. If vasculogenic mechanisms of placentation are similar to those reported for tumor invasion there are at least two forces driving the acquisition of the new blood supply. Maternal endothelial cells are recruited toward the placenta and concomitantly, placental cells migrate toward proliferating maternal vessels.
[0153] The factors that pr...
example 3
General Procedure for the Production of Peptides
[0180] This example illustrates the general methods used for the production of peptides of the invention.
[0181] Intact full length human prolactin (hPL), human growth hormone (hGH), human growth hormone variant (hGH-v), human placental lactogen (hPL) and 16K N-terminal fragment of human prolactin (16K hPRL), growth hormone (16K hGH), growth hormone variant (16 hGH-V) and placental lactogen (16K hPL) were produced as described below.
[0182] The coding sequences, antisense sequences, and amino acid sequences of the intact hormones and 16K N-terminal peptides are listed in sequences identified as SEQ ID NO:1-30 as follows.
hPRL (Met−1Cys199)(SEQ ID NO:1)ATGTTGCCCA TCTGTCCCGG CGGGGCTGCC CGATGCCAGG TGACCCTTCG AGACCTGTTT 60GACCGCGCCG TCGTCCTGTC CCACTACATC CATAACCTCT CCTCAGAAAT GTTCAGCGAA120TTCGATAAAC GGTATACCCA TGGCCGGGGG TTCATTACCA AGGCCATCAA CAGCTGCCAC180ACTTCTTCCC TTGCCACCCC CGAAGACAAG GAGCAAGCCC AACAGATGAA TCAAAAAGAC240TTTCTGAGCC TGAT...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com