Knee joint prosthesis and hyaluronate compositions for treatment of osteoarthritis
a technology of hyaluronate compositions and joints, applied in the field of therapies, can solve the problems of limiting patient's mobility, reducing and limiting the mobility of the knee joint, so as to reduce the number of injections, improve the effect of lubrication duration, and reduce pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example no.1
Example No. 1 Meniscal Wafer Manufacturing
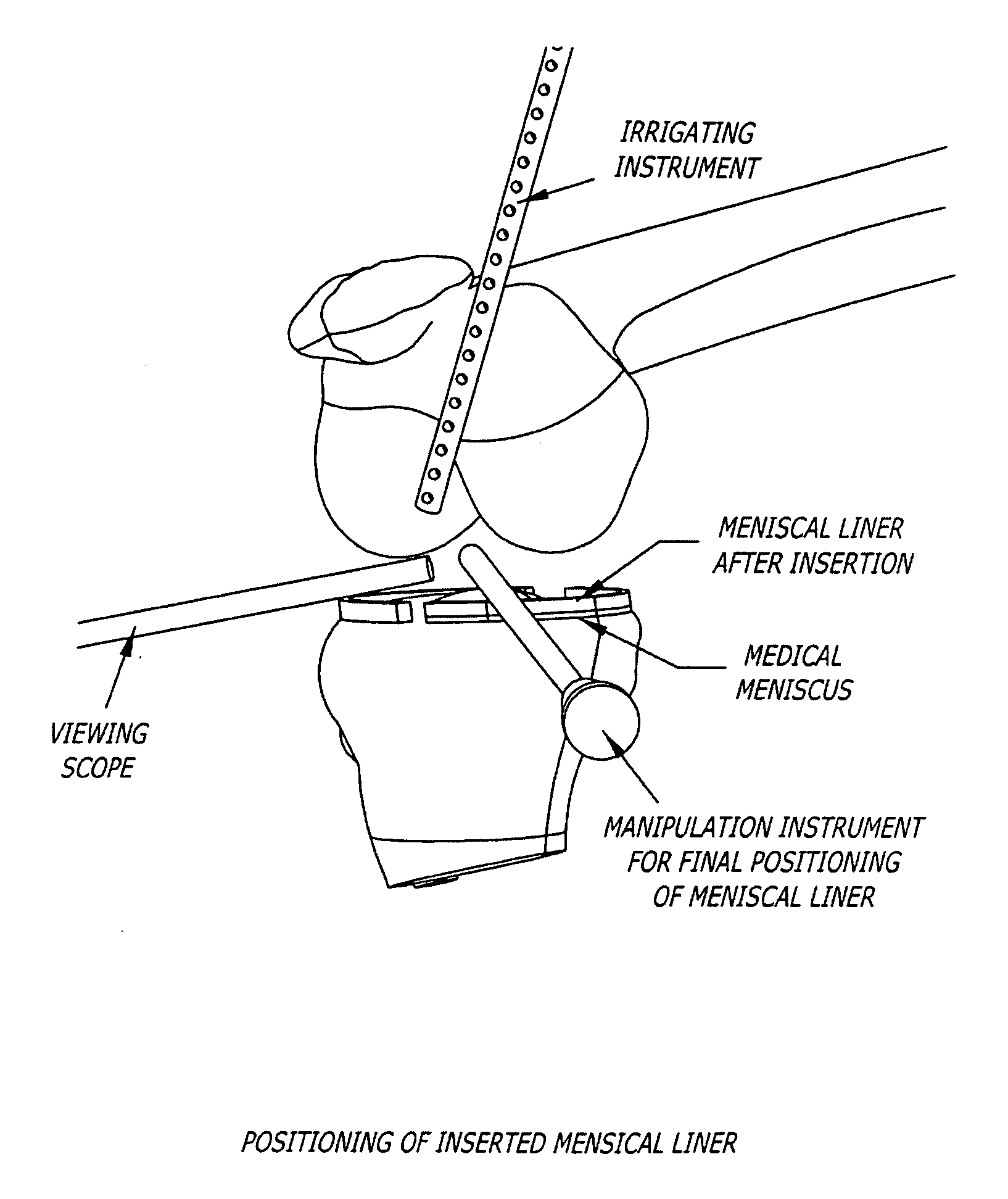
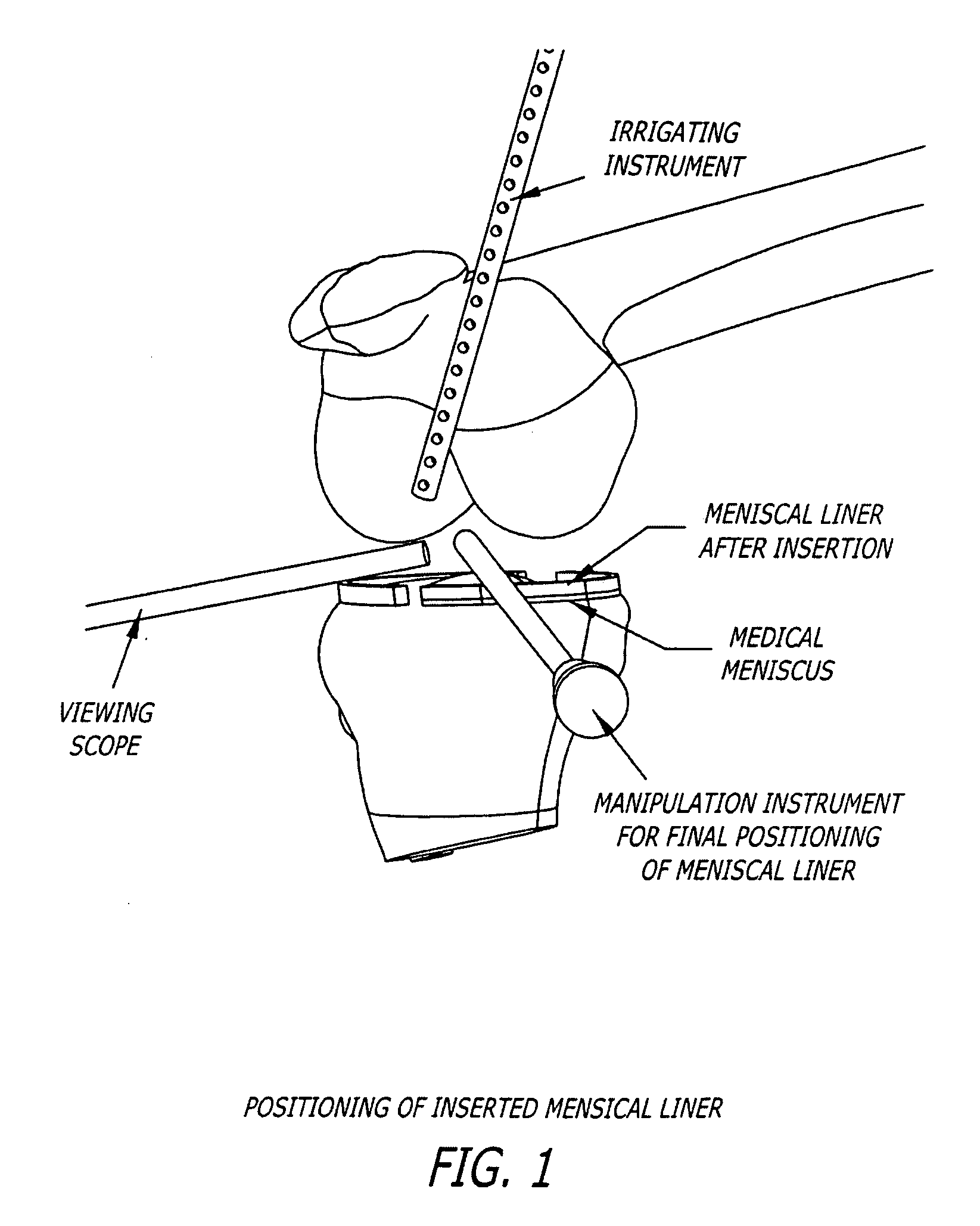
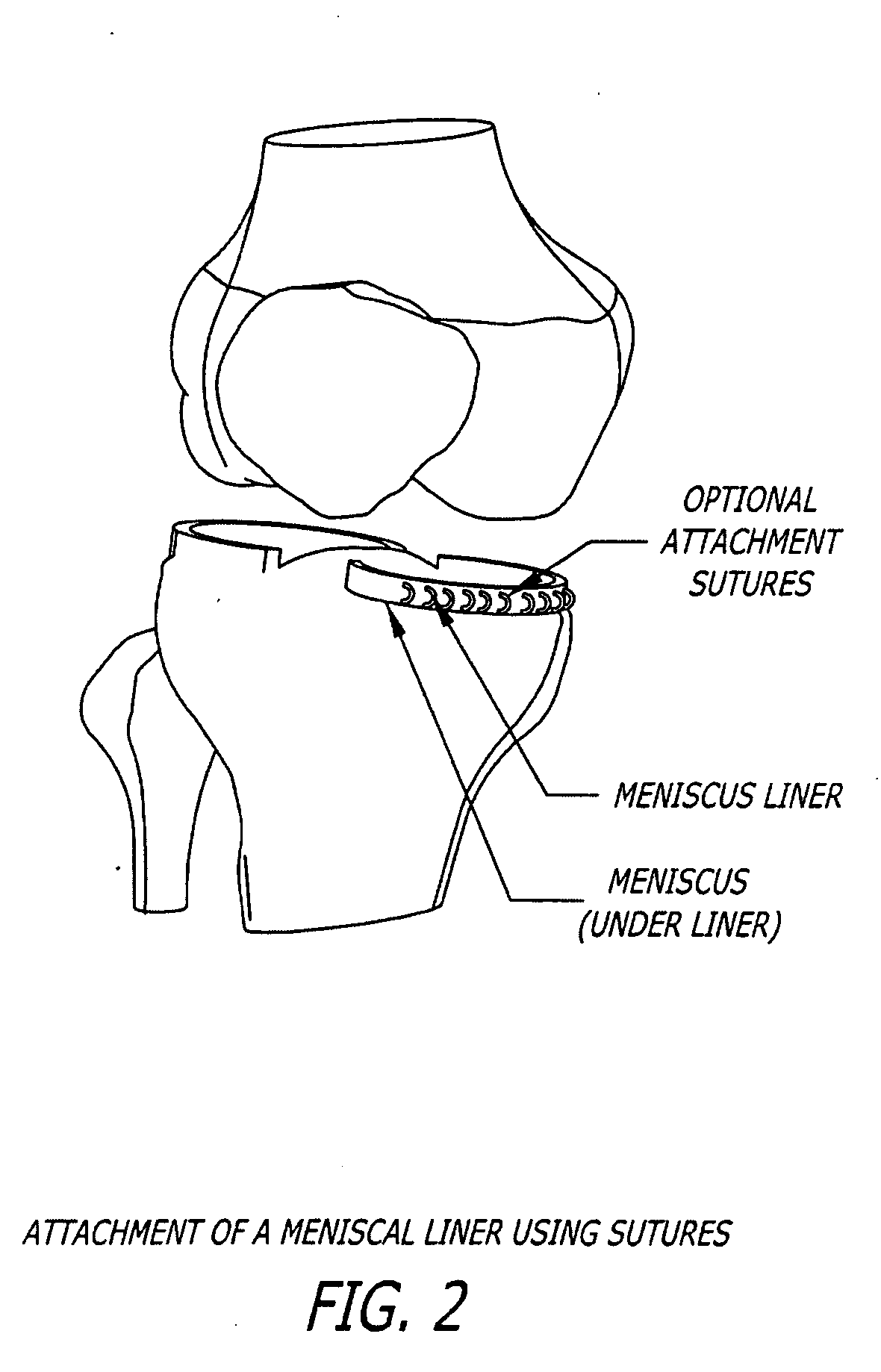
[0130] A meniscal wafer (MW) is a medical device implanted via an arthroscope into the knee joint space (see FIG. 1) to act as a support and bearing wear surface between the femoral condyle and the meniscus / tibial plateau. It is for (1) use in partial or total meniscectomy to supplement or provide meniscus function and articulating surface (to minimize joint degradation), (2) use in place of partial or total meniscectomy (to delay joint degradation), (3) use after arthroscopic clean-up of osteoarthritis (OA) joint to provide fresh articulating surface and improve biomechanics (to delay total knee replacement, for example), and / or (4) to adjust the alignment of the joint though height supplementation. Meniscal wafers can be used on the medial, lateral or both sides of one or both joints. Meniscal wafers can (i) cover the meniscus (only), (ii) cover the tibial plateau (only) or (iii) cover both. FIG. 5 (B-D) shows placement of a meniscal wafer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com