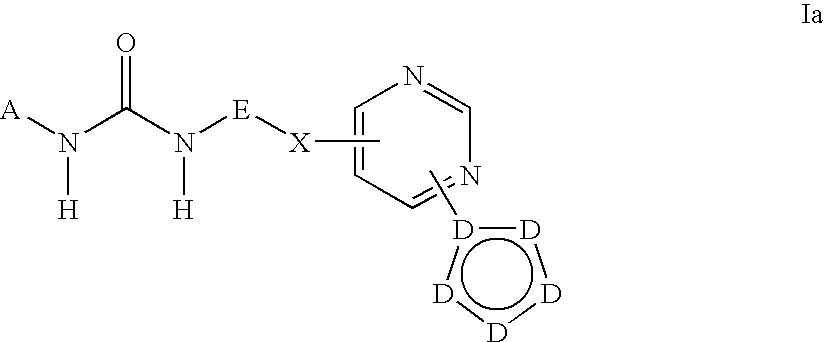
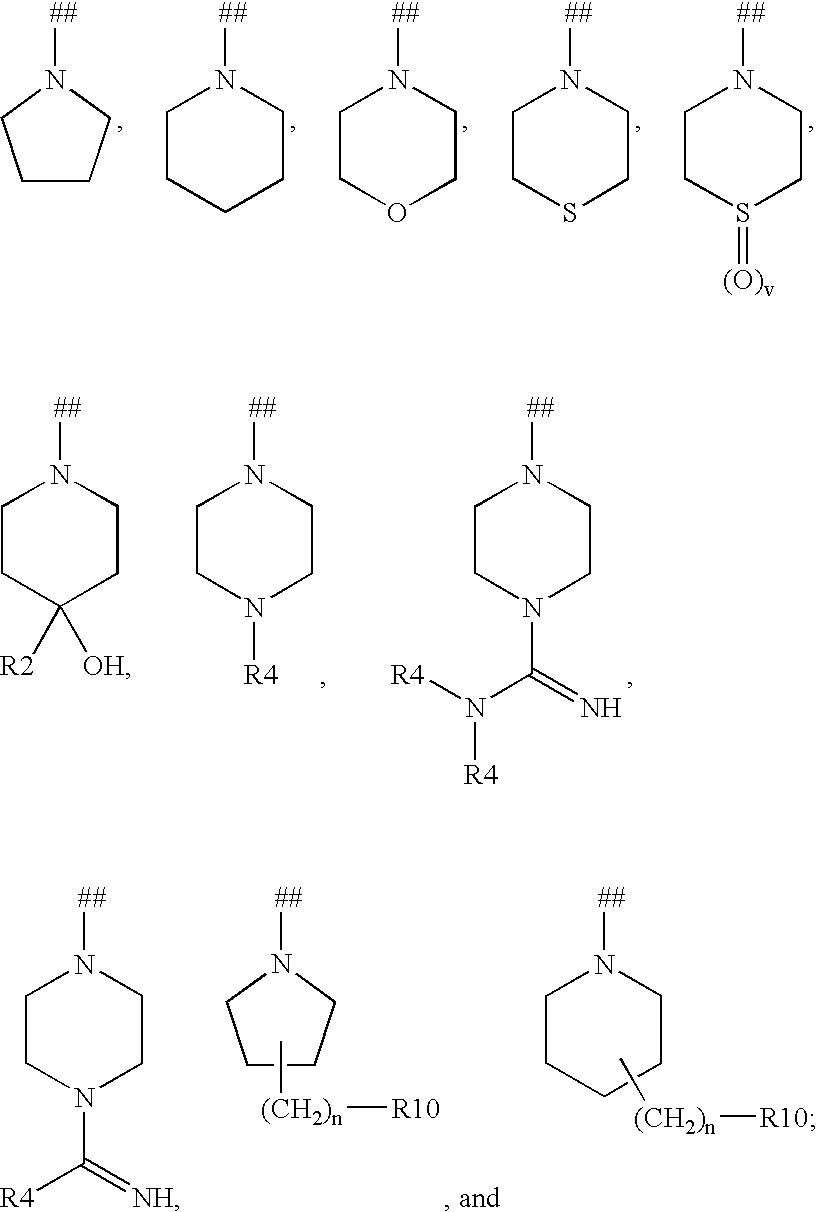
Kinase inhibitors useful for the treatment of myleoprolific diseases and other proliferative diseases
a technology of kinase inhibitors and proliferative diseases, applied in the field of kinase inhibitors and modulator compounds, can solve the problems of molecularly targeted small therapies that target c-kit mutations that remain elusiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0108]To a stirring solution of the carboxylic acid (0.24 mmol) and TEA (1.2 mmol) in 1,4-dioxane (4.5 mL) at RT was added DPPA (0.29 mmol). After stirring for 0.5 h at RT, the appropriate amine (0.71 mmol) was added and the reaction was stirred with heating at 100° C. for 2 h. The reaction was cooled to RT, diluted with brine (15 mL) and extracted with EtOAc (3×30 mL). The combined organic layers were dried (MgSO4) and concentrated. The residue was purified by chromatography to afford the target compound.
example a1
[0109]4-Fluoro-2-methyl-phenol (25 g, 0.2 mol) was added to a solution of sodium hydroxide (9.7 g, 0.24 mol) in water (160 mL) and the resultant solution was cooled to 0° C. Methyl chloroformate (24.2 g, 0.26 mol) was added dropwise at 0° C. At the completion of the reaction, the pH was adjusted to pH 8 with saturated aqueous Na2CO3 and then mixture was extracted with ethyl acetate (3×300 mL). The combined organic extracts were washed with water and brine, dried (MgSO4) and were concentrated under reduced pressure to provide carbonic acid 4-fluoro-2-methyl-phenyl ester methyl ester (30 g, 82% yield). 1 H NMR (300 MHz, DMSO-d6): δ 7.22-7.13 (m, 2 H), 7.05 (m, 1H), 3.81 (s, 1H), 2.12 (s, 3H).
[0110]To a solution of carbonic acid 4-fluoro-2-methyl-phenyl ester methyl ester (15 g, 81.5 mmol) in conc. sulfuric acid (100 mL) at 0° C. was added powdered KNO3 (8.3 g, 82.2 mmol) in several portions. The reaction mixture was stirred for 1 hour at 0° C. and was then poured into ice water and ex...
example a2
[0114]Methyl chloroformate (77.3 g, 0.82 mol) was added dropwise to a −10° C. solution of 2-chloro-4-fluorophenol (100 g, 0.68 mol) and sodium hydroxide (32.8 g, 0.82 mol) in water (550 mL). After complete addition, the precipitated solid was collected by filtration and washed with water to give 2-chloro-4-fluorophenyl methyl carbonate (110 g, 79% yield). 1H NMR (300 MHz, DMSO-d6): δ 7.62 (dd, J=8.1, 2.7 Hz, 1H), 7.50 (dd, J=9.0, 5.4 Hz, 1H), 7.30 (td, J=8.1, 3.0 Hz, 1H), 3.86 (s, 3H); MS (ESI) m / z: 205.2 (M+H+).
[0115]To a suspension of 2-chloro-4-fluorophenyl methyl carbonate (110 g, 0.54 mol) in conc. H2SO4 (50 mL) was slowly added a mixture comprised of conc. H2SO4 (40 mL) and fuming HNO3 (40.8 mL, 0.89 mol). The resultant mixture was stirred for 30 min at 0° C. The reaction mixture was poured into ice water and the precipitated solid was collected by filtration and washed with water to rive 2-chloro-4-fluoro-5-nitrophenyl methyl carbonate (120 g, 90% yield). 1H NMR (400 MHz, DM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| regioisomers | aaaaa | aaaaa |
| oxidative stress | aaaaa | aaaaa |
| kinase domain catalytic activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com