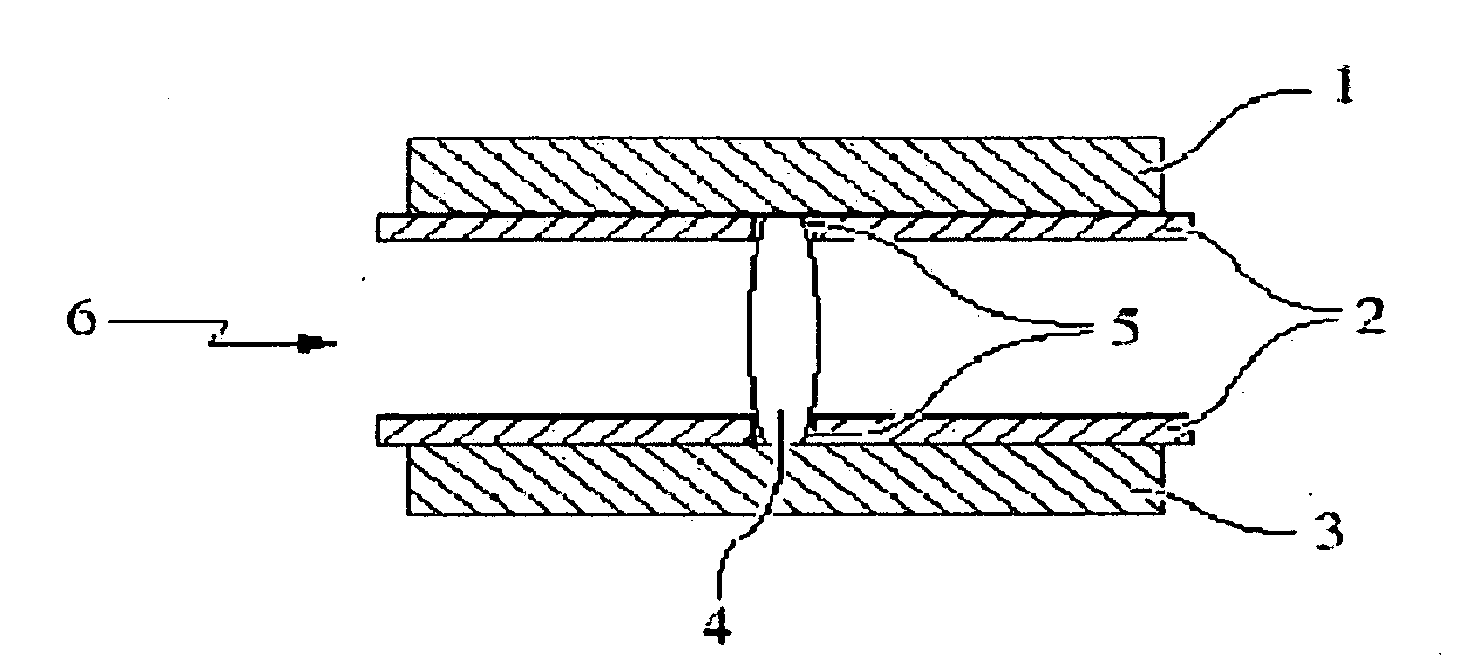
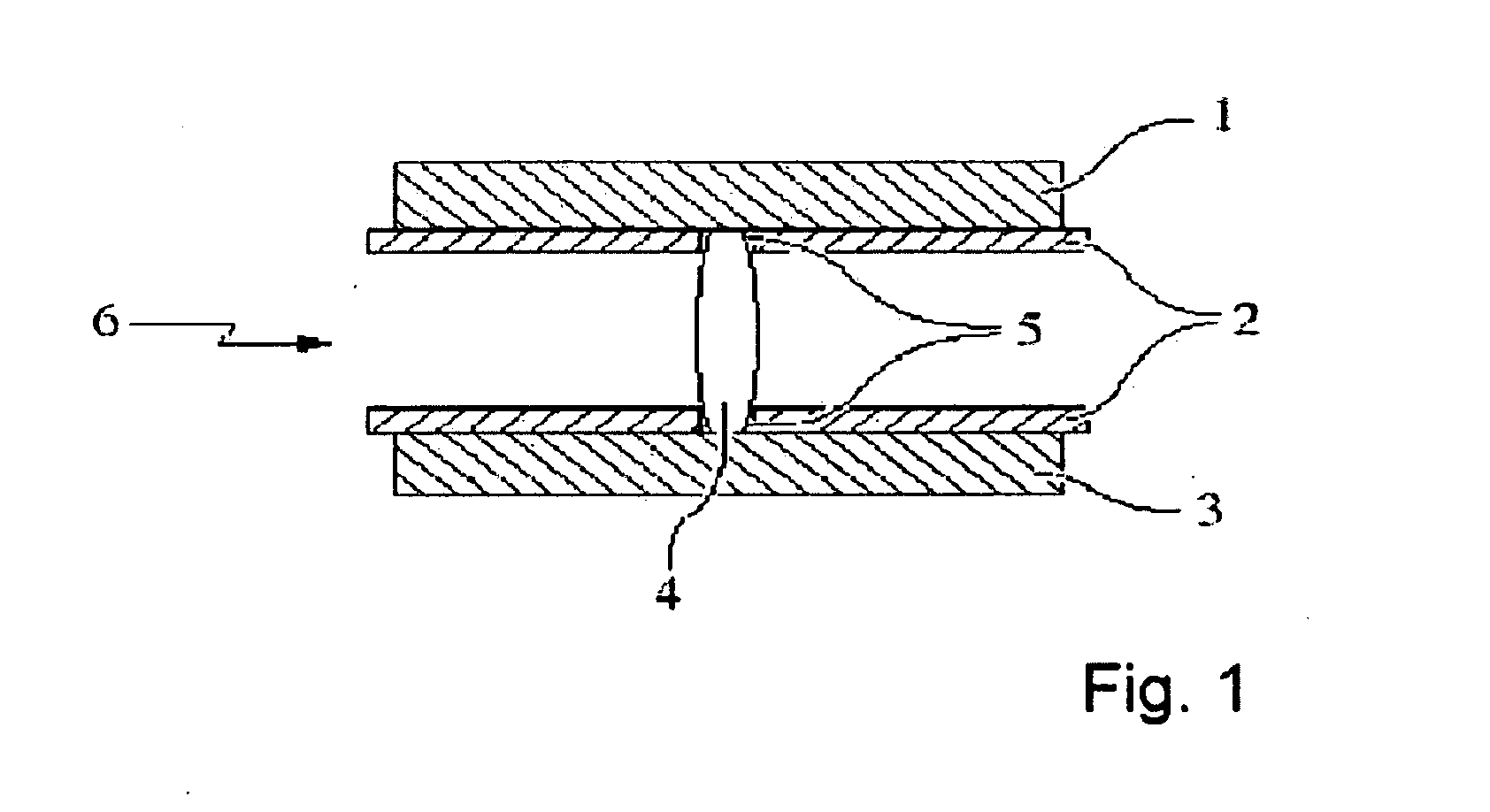
Process for the production of an abuse-proofed dosage form
a technology of dosage form and production process, which is applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of inability to actually perform safe administration and the inability to extract active ingredients from there, etc., and achieve the effect of intense negative effect and no negative
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0159]
Per tabletComplete batchTramadol HCl100.0mg1495.0gPolyethylene oxide,167.8mg2508.6gMW 7 000 000(Polyox WSR 303 from Dow)Hydroxypropylmethylcellulose33.5mg500.8g(Hypromellose 100 000 mPa)Butyihydroxytoluene (BHT)0.2mg3.0gTotal mass300.5mg4507.4g
[0160]The stated quantity of BHT was dissolved in ethanol (96%), such that a 7.7% (mass / mass) ethanolic solution was obtained. This was mixed initially with 150 g of polyethylene oxide in a high speed mixer for 30 minutes and then the remaining quantity of polyethylene oxide was added and stirring continued for a further 30 minutes. The composition was dried for 12 h at 40° C.
[0161]All the further components were added and mixed for 15 min in a free-fall mixer. The powder mixture was divided between moulds, each having a diameter of 13 mm and a depth of 6 mm. Using a syringe with cannula, the mixture was suspended in each case in 0.5 ml of 96% ethanol and then in each case combined with 0.5 ml of distilled water After 24 hours swelling t...
example 2
[0165]
Powder mixtureComplete batchPer tabletTramadol HCl100.1g100mgPolyethylene oxide300.0g299.7mgMW 5000 000(Polyox WSR Coagulant,from Dow),Hydroxypropylmethylcellulose50.05g50.0mg(Hypromellose 100 000 mPa)Butylhydroxytoluene (BHT)0.25g0.25mgFoam0.250g0.25mgHydroxypropylmethylcellulose(Hypromellose 100 000 mPa)Dist. water49.8g
[0166]The powder mixture was first produced as stated in Example 1.
[0167]The foam was produced by dissolving the stated quantity of Hypromellose in distilled water. A foam was then produced using a high performance homogeniser (IKA Ultraturrax 25 Basic) by stirring initially for 2 minutes at level 1, then for 2 minutes with a mixer / granulator at level 2 and finally for 3 minutes at level 3. The powder mixture was slowly added to the foam with constant stirring in a mixer (Kenwood Major Classic 25 Basic).
[0168]The granulated mixture was then dried for 24 hours—at 40° C. and, after being passed through a screen (from Frewitt, model GLA-A-ORV) with 1 mm orifices,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Force | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com