Dispersed metal sulfide-based catalysts
a technology of dispersed metal sulfide and catalyst, which is applied in the direction of catalyst activation/preparation, metal/metal-oxide/metal-hydroxide catalyst, physical/chemical process catalyst, etc., can solve the problem of increased cost, increased dispersed catalytic solids or oil-soluble compounds, and increased activity of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
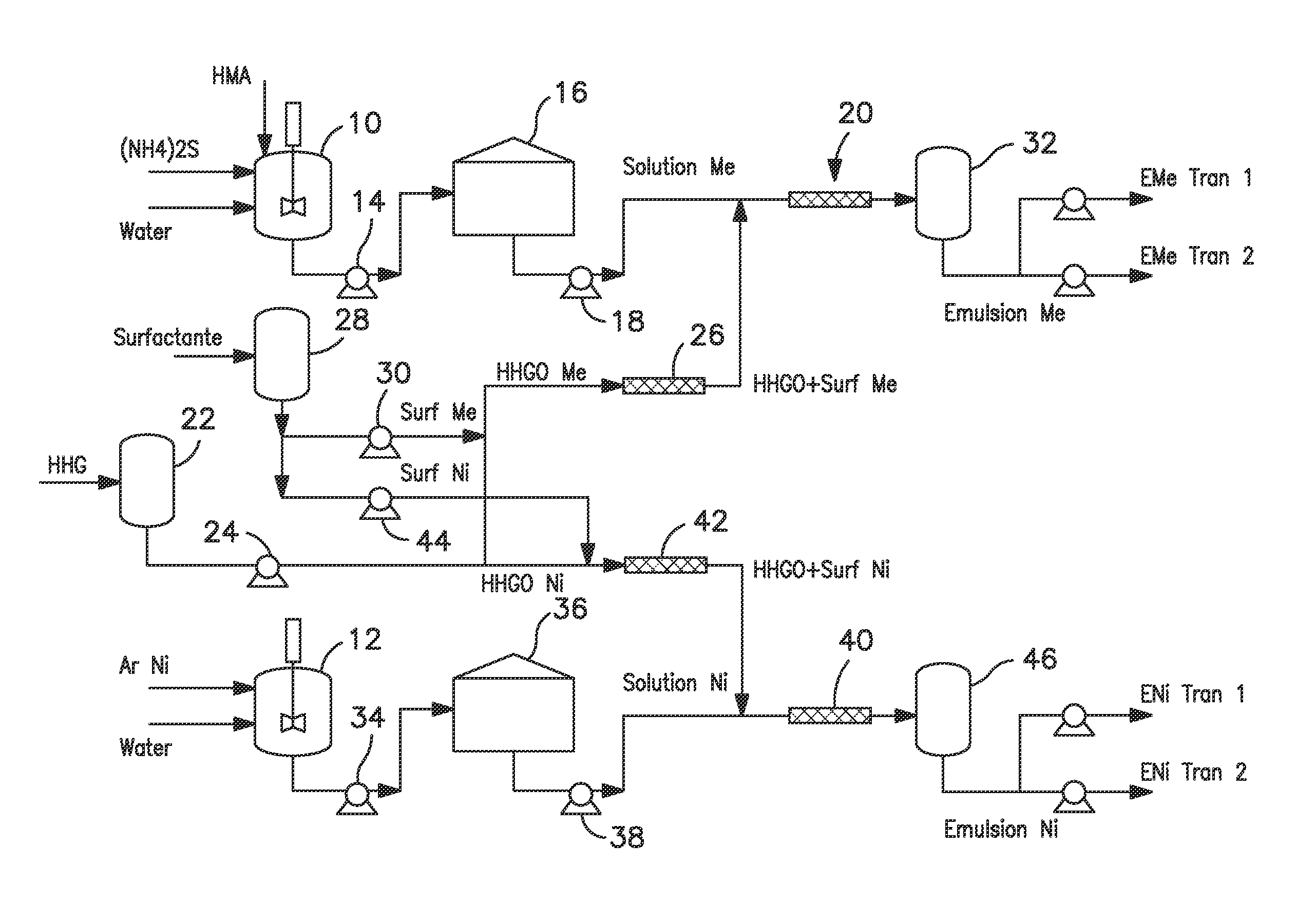
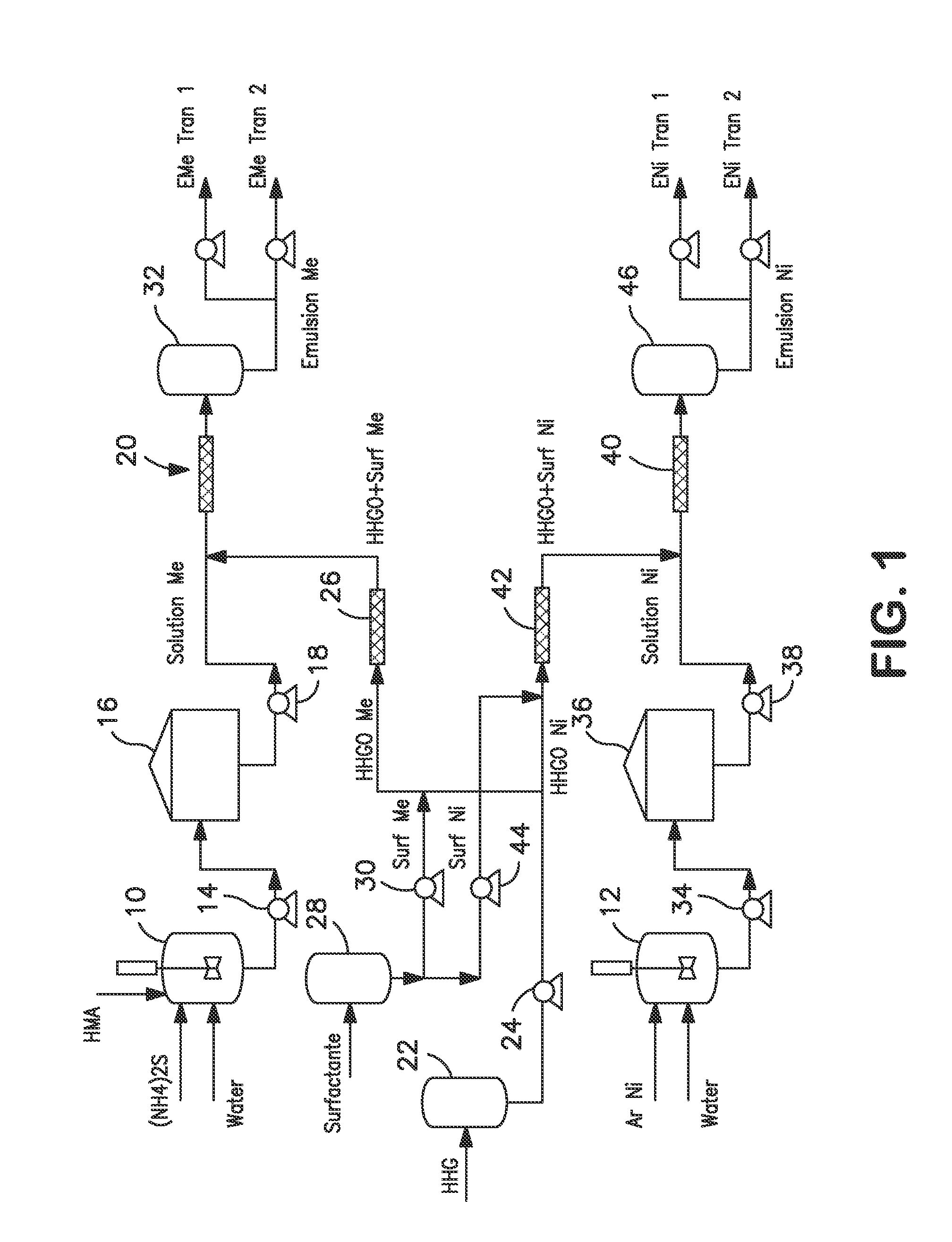
[0047]An aqueous solution containing catalytic precursors was prepared from ammonium heptamolybdate (AHM) [(NH4)6Mo7O24.4H2O]. The aqueous solution was prepared having a concentration of 1-14 wt % in sour water. For this example, the concentration was 10 wt %. The sour water was prepared using ammonium sulfide [(NH4)2S] at a concentration of 0.1-14% wt. For this example, the concentration was 0.2 wt %. The AHM reacts with ammonium sulfide to generate soluble oxy-sulfurs.
[0048]A w / o emulsion was prepared using a ratio of mass of surfactant to total mass of the emulsion (γ) between 0 and 0.01 and a ratio by mass of oil to combined mass of aqueous solution and oil ({acute over (α)}) of between 0.7 and 0.94. The aqueous solution and the oil phase can be formed into an emulsion without surfactants (only natural surfactants contained in the oil, such as resins or naphthenic acids contained in HVGO), or using a non-ionic surfactant with an Hydrophilic-Lipophilic Balance ...
example 2
[0053]A water-oil emulsion was prepared from nickel acetate [Ni(CH3COO)2.H2O] aqueous solution (7-14% wt) and HVGO or HHGO, with or without non-ionic surfactant. The relationships γ and {acute over (α)} were the same as for the w / o molybdenum emulsion, between 0-0.01 and 0.70-0.94 respectively. Table 3 shows the component preferred ranges of the emulsion and their concentration in the mixture, as well as specific values for this example.
TABLE 3Component(% wt / wt)[Ni (CH3 COO)2•H2O] aqueous 6.00-30.00solution 7-14%(0.2%)Surfactant 0-1.00HVGO / HHGO73.00-90.70[Ni (CH3COO)2•H2o] aqueous solution26.31Surfactant0.90HVGO / HHGO72.78
[0054]The preparation parameters and control quality methods applied for the water-oil nickel emulsion coincide with those that have been applied for the molybdenum emulsion. FIGS. 4a and 4b show the droplet diameter size distribution and a digitalized optical image of the nickel emulsion, respectively. The droplets have an average diameter of 2.7 μm...
example 3
Bi-Metallic Catalyst Synthesis
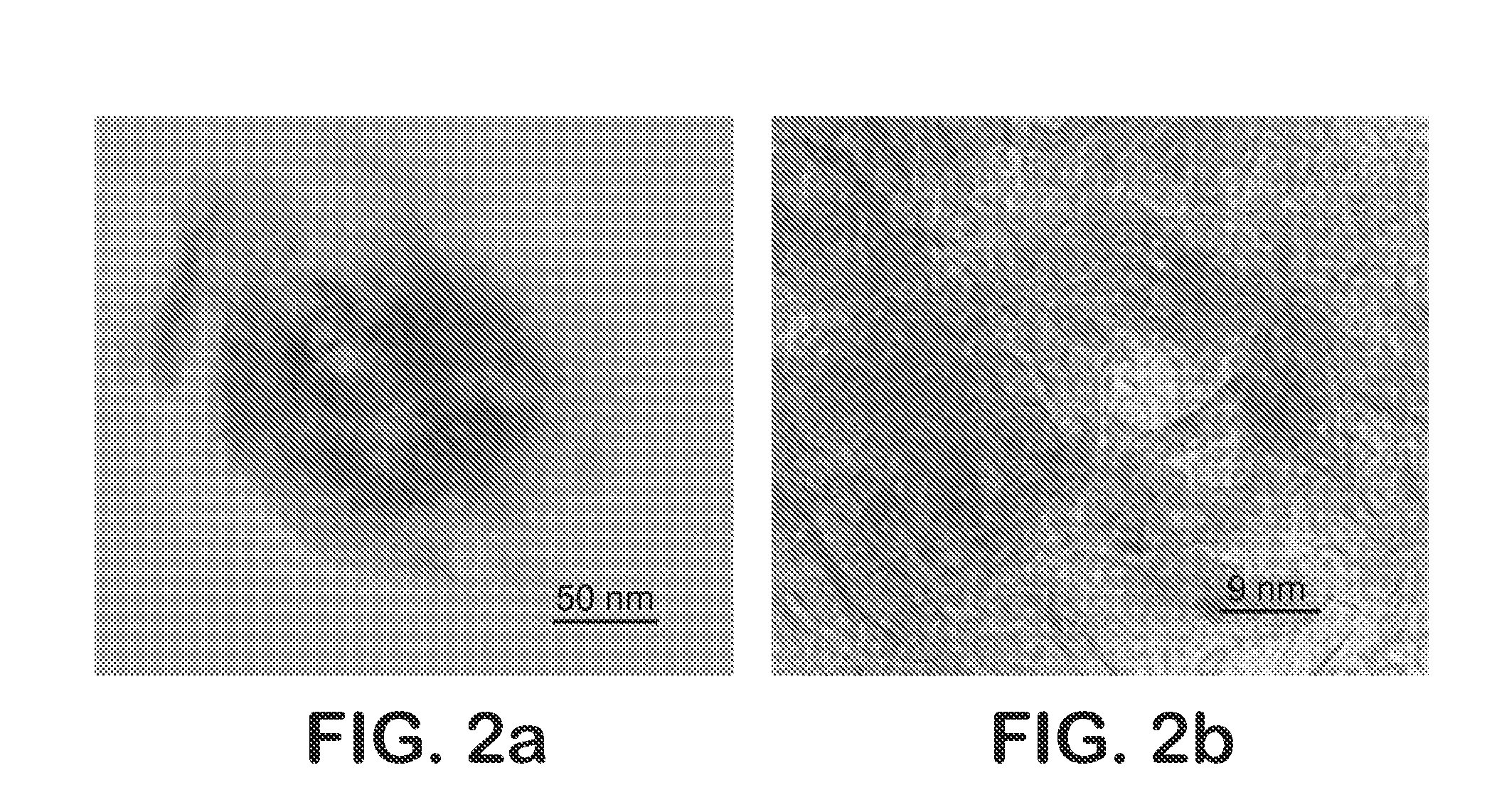
[0056]The synergy effect of NiMoS supported catalyst is well known. However, this effect is not evident in dispersed catalyst systems. Due to this fact, thermal decomposition of the simultaneous nickel and molybdenum water-oil emulsions was tested. The ratio of Ni / Ni+Mo was changed from 0 to 1 and HDS activity measured at different points in this range. FIG. 5 shows HDS activity results for solids obtained from simultaneous thermal decomposition. It is clear that Ni enhances the performance of Mo catalyst and the maximal synergistic effect was found at a ratio of 0.1. HTEM results evidenced particle size in the range of the previous described particles (Mo—S and Ni—S).
[0057]It should be appreciated that a new catalyst system has been provided in accordance with invention which produces fine or ultra-dispersed catalyst particles and thereby greatly enhances hydroconversion activity of the catalyst when exposed to a suitable feed stock. It should also be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com