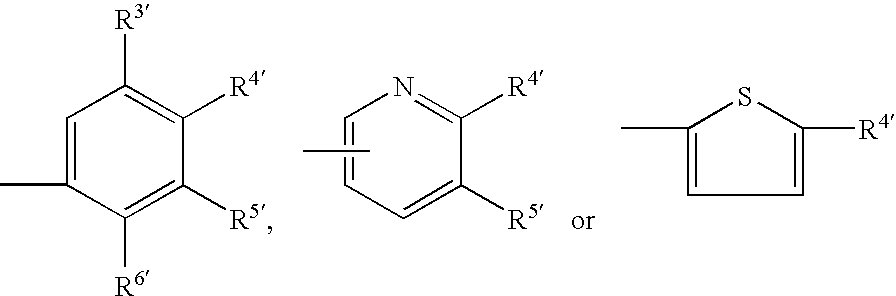
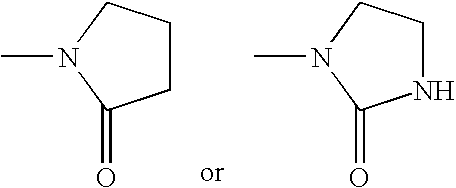
Inhibition of p38 kinase using symmetrical and unsymmetrical diphenyl ureas
a diphenyl urea and kinase technology, applied in the field of aryl ureas, can solve the problems of compound structure-related toxicity, no marketed pharmaceutical agent is able to prevent or slow cartilage loss,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0131]All reactions were performed in flame-dried or oven-dried glassware under a positive pressure of dry argon or dry nitrogen, and were stirred magnetically unless otherwise indicated. Sensitive liquids and solutions were transferred via syringe or cannula, and introduced into reaction vessels through rubber septa. Unless otherwise stated, the term ‘concentration under reduced pressure’ refers to use of a Buchi rotary evaporator at approximately 15 mmHg.
[0132]All temperatures are reported uncorrected in degrees Celsius (° C.). Unless otherwise indicated, all parts and percentages are by weight.
[0133]Commercial grade reagents and solvents were used without further purification. Thin-layer chromatography (TLC) was performed using Whatman® pre-coated glass-backed silica gel 60A F-254 250 μm plates. Visualization of plates was effected by one or more of the following techniques: (a) ultraviolet illumination, (b) exposure to iodine vapor, (c) immersion of the plate in a 10% solution o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com