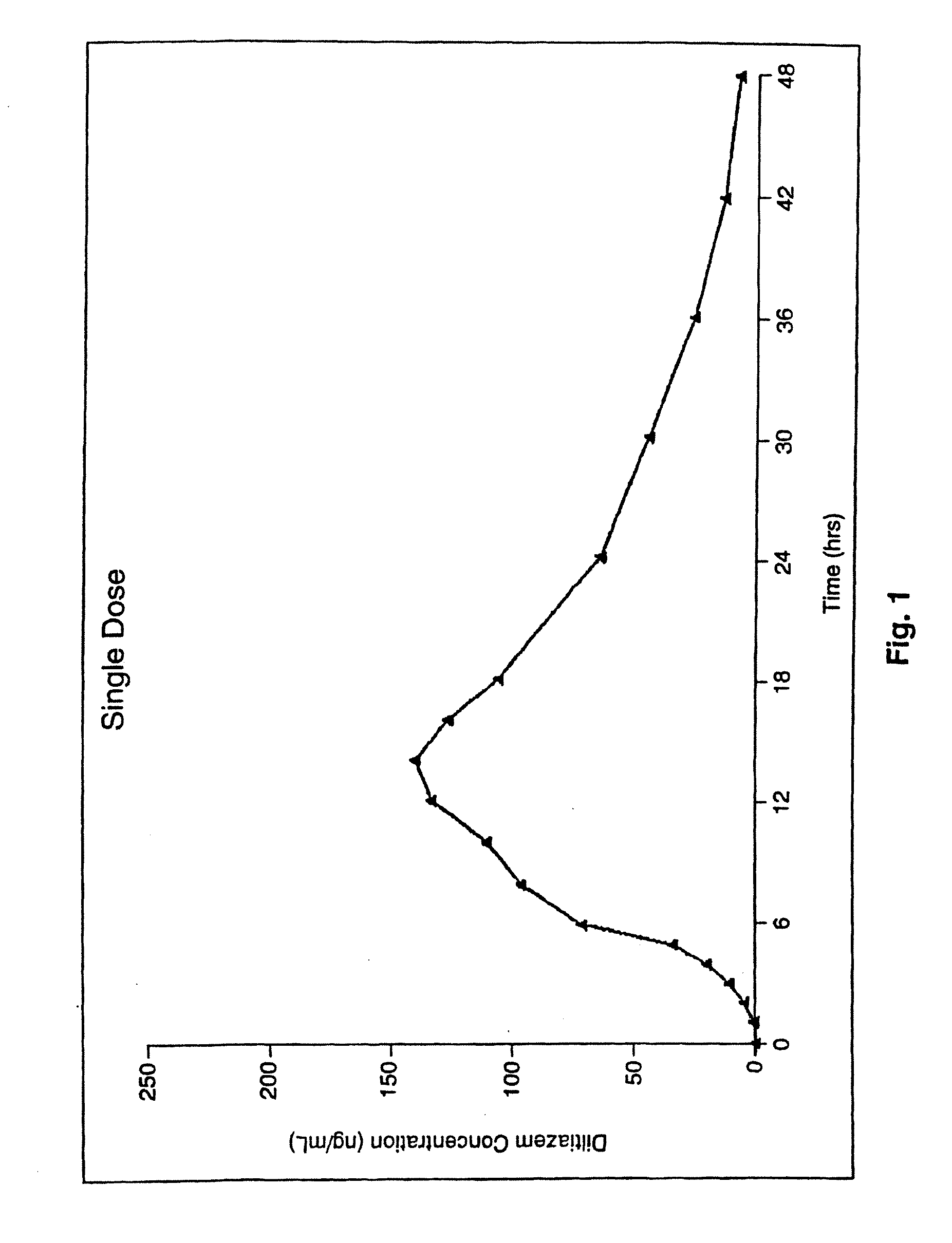
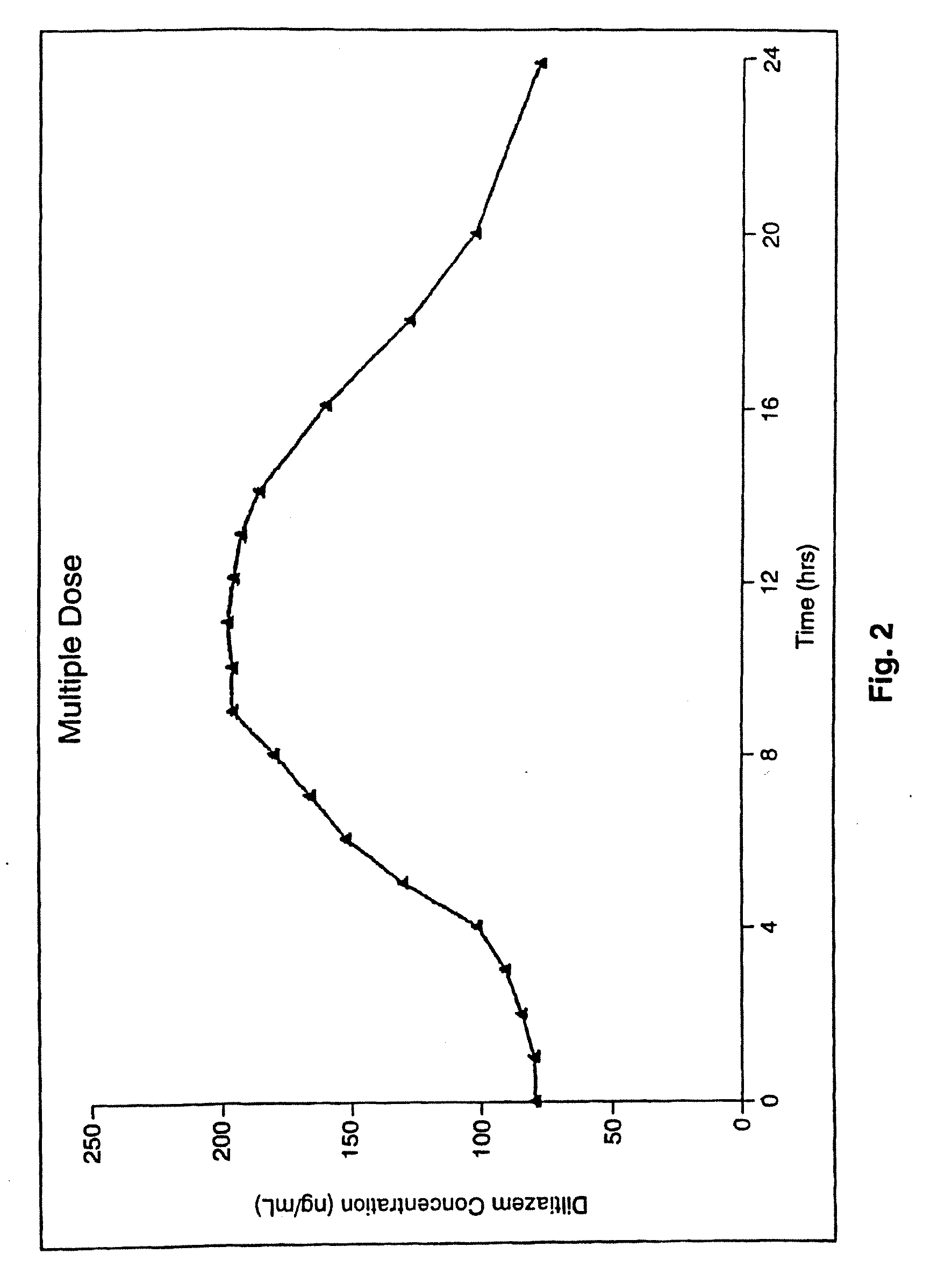
Chronotherapeutic diltiazem formulations and the administration thereof
a technology of diltiazem and formulations, which is applied in the direction of biocide, drug compositions, cardiovascular disorders, etc., can solve the problems of inability to support the authors' conclusions, inability to lead to reliable conclusions, and the immediate release portion of the dosage in the order of 15% is not desirable for evening administration, so as to achieve greater auc and cmax, the effect of increasing bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Example 3
[0140]
StrengthStrength120 mg capsule180 mg capsule(1)120.00(1)180.00(2)13.63-16.18(2)20.44-24.27(3) 1.7-3.41(3)2.56-5.11(4)11.92-13.63(4)17.88-20.44(5)0.852-4.26 (5)1.278-6.388(6)0.852-8.52 (6)1.278-12.78(7)0.256-0.511(7)0.383-0.767(8)0.511-1.02 (8)0.7665-1.533 (9)0.0170-0.0426(9)0.0256-0.0639(10) 0.017-0.0256(10) 0.0255-0.383 (11) 11.92-18.74(11) 17.886-28.106(12) 0 (12) 0
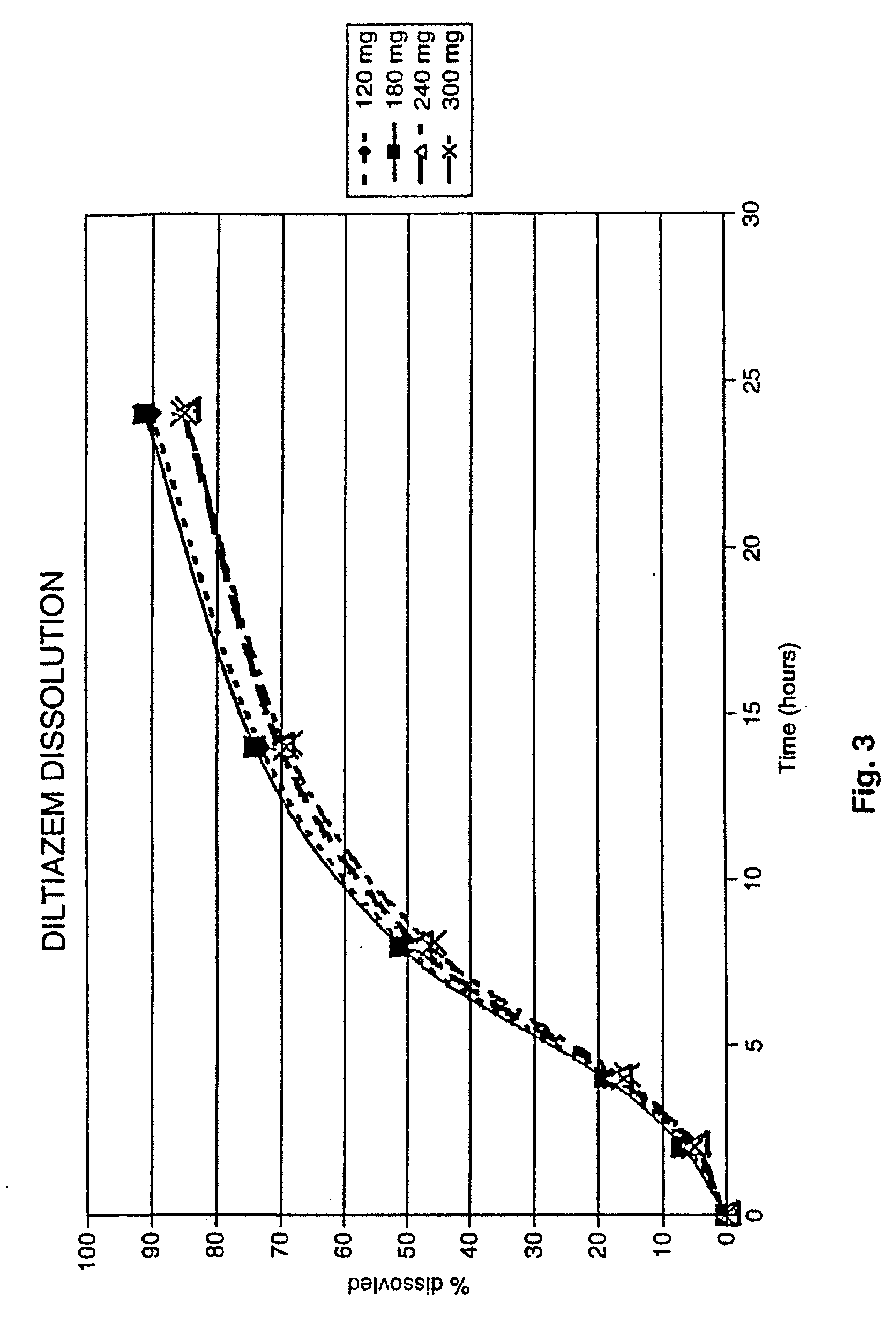
[0141]240 mg, 300 mg, 360 mg and 420 mg strength preparations in capsule form of Diltiazem (as the HCl salt) were also prepared having the same percentages.
[0142]They provide the release patterns shown in FIG. 3. The dissolution profiles of all of the strengths were generated from biobatches of capsules using Apparatus 1 (baskets) at 100 RPM in 900 ml of water in accordance with USP 23.
[0143]Less than 20% of the formulation is dissolved after about four hours (for example between about 16%-21%) with less than about 10% dissolved in the first two hours (for example between about 4%-about 8%). Less than a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration Cmax | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com