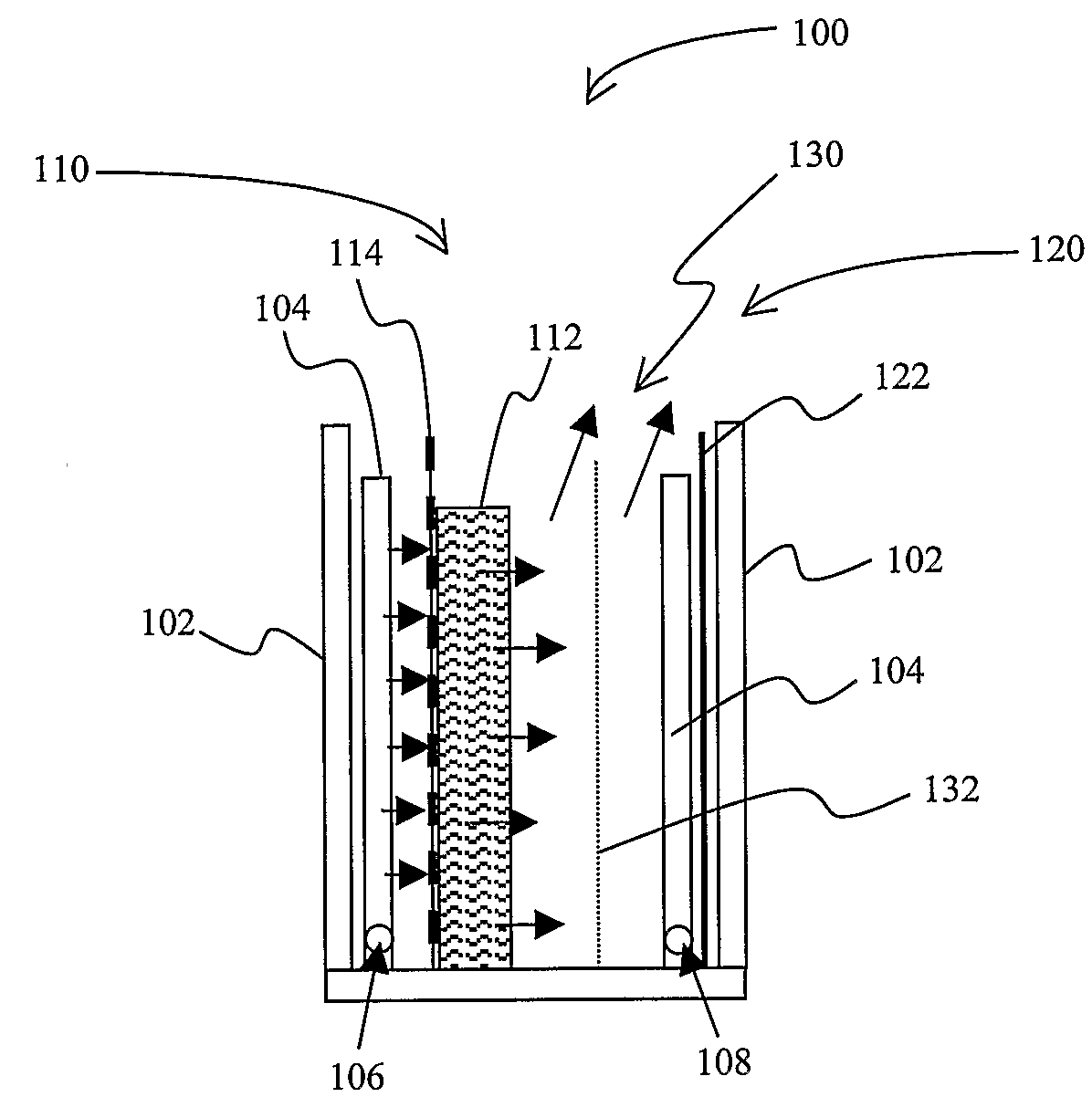
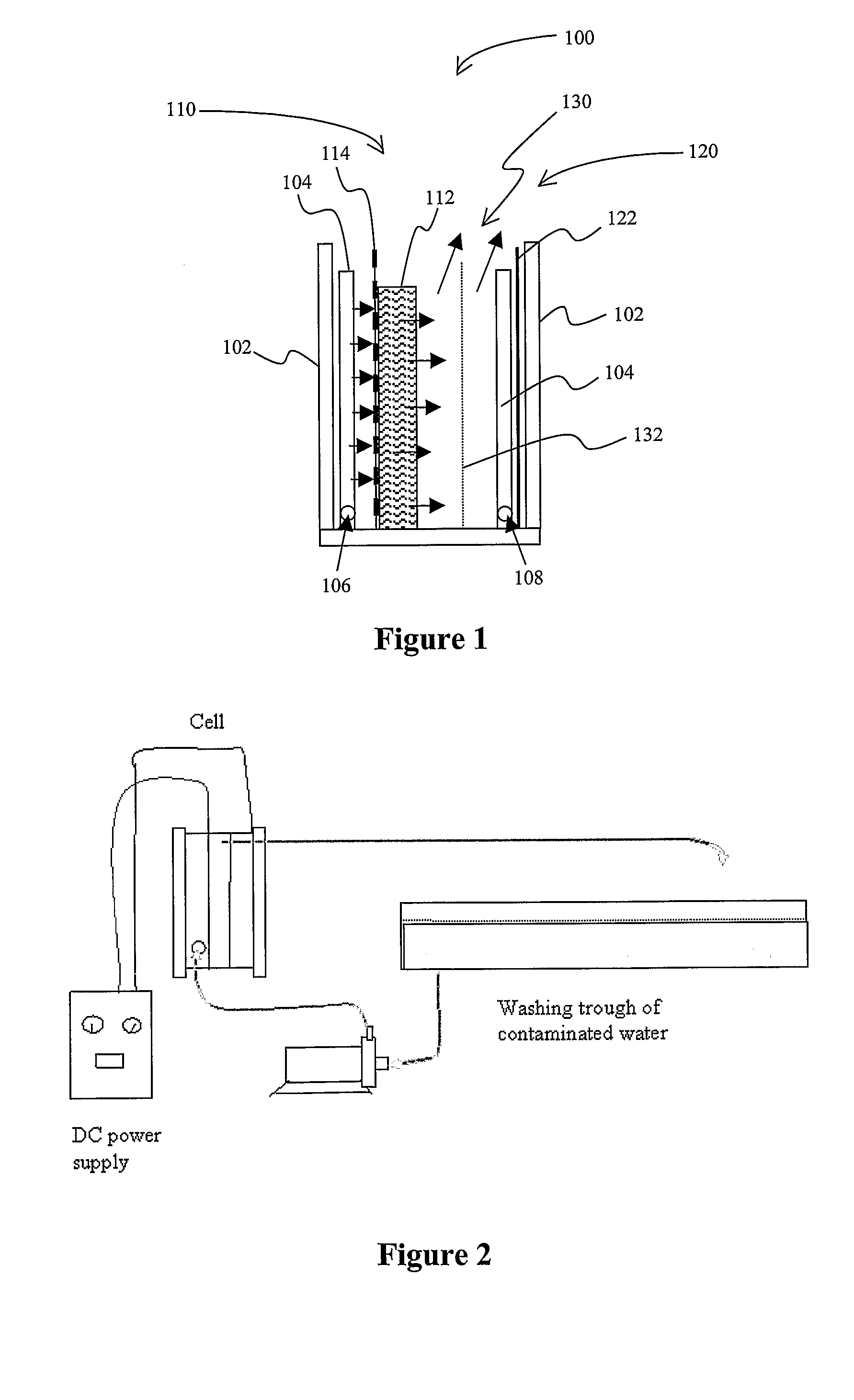
Methods and Apparatus for Generating Oxidizing Agents
a technology of oxidizing agent and electrochemical generation, which is applied in the production of electrolytic organic products, electrolysis components, and the nature of treatment water, etc. it can solve the problems of oxidizing anode oxidation damage, hypochlorite producing a relatively bad taste in the treated water, and requiring an experienced operator to dosing, so as to reduce and/or destroy a contaminant, and eliminate oxidative damage to the anode
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0039]Acid violet 7B, a common triphenylene dye identified by reference to the Color Index as number 42745 was added to deionized water at a concentration of 100 milligrams per liter. Deionized water was made conductive with addition of 2 grams per liter of sodium sulfate. This solution was pumped through the anode compartment described above with the current off. The dye concentration remained the same. A 3.0 amps current was then applied to the cell and the solution pumped through at the rate of 30 ml per minute. Remarkably, the solution was completely decolorized as it exited the cell. The experiment was repeated with Allura Red AC, a common foodstuff dye identified as CI # 16035, and later with Brilliant Green #42040 in the Color Index with the same results.
example 2
[0040]Perchlorobiphenyl (PCB) was extracted from transformer oil by emulsification with a commercially available emulsifying agent LA8 sold by Imperial Chemical Industries Ltd. The PCB extract was added to water producing a solution that was found by analysis to be 12 mg / liter of PCB. The solution was pumped through the anode compartment at no current, and the exiting solution was found to be 11 mg / liter of PCB. When the current was turned on, the solution leaving the anode compartment contained no detectable levels of PCB.
example 3
[0041]DDT (1,1-Bis-(p-chlorophenyl)-2,2,2-trichloroethane) extracted from an old disused spray containing the organophosphate pesticide Methidathion (O,O-Dimethyl S-(5-methoxy-1,3,4-thiadiazolinyl-3-methyl)dithiophosphate) was added to the test solution producing a solution which on analysis gave 53 ppm of DDT. After passing through the anode compartment without electrolysis, the concentration was found to be 54 ppm, while no DDT was detected and after electrolysis of the solution in the electrolytic anode compartment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com