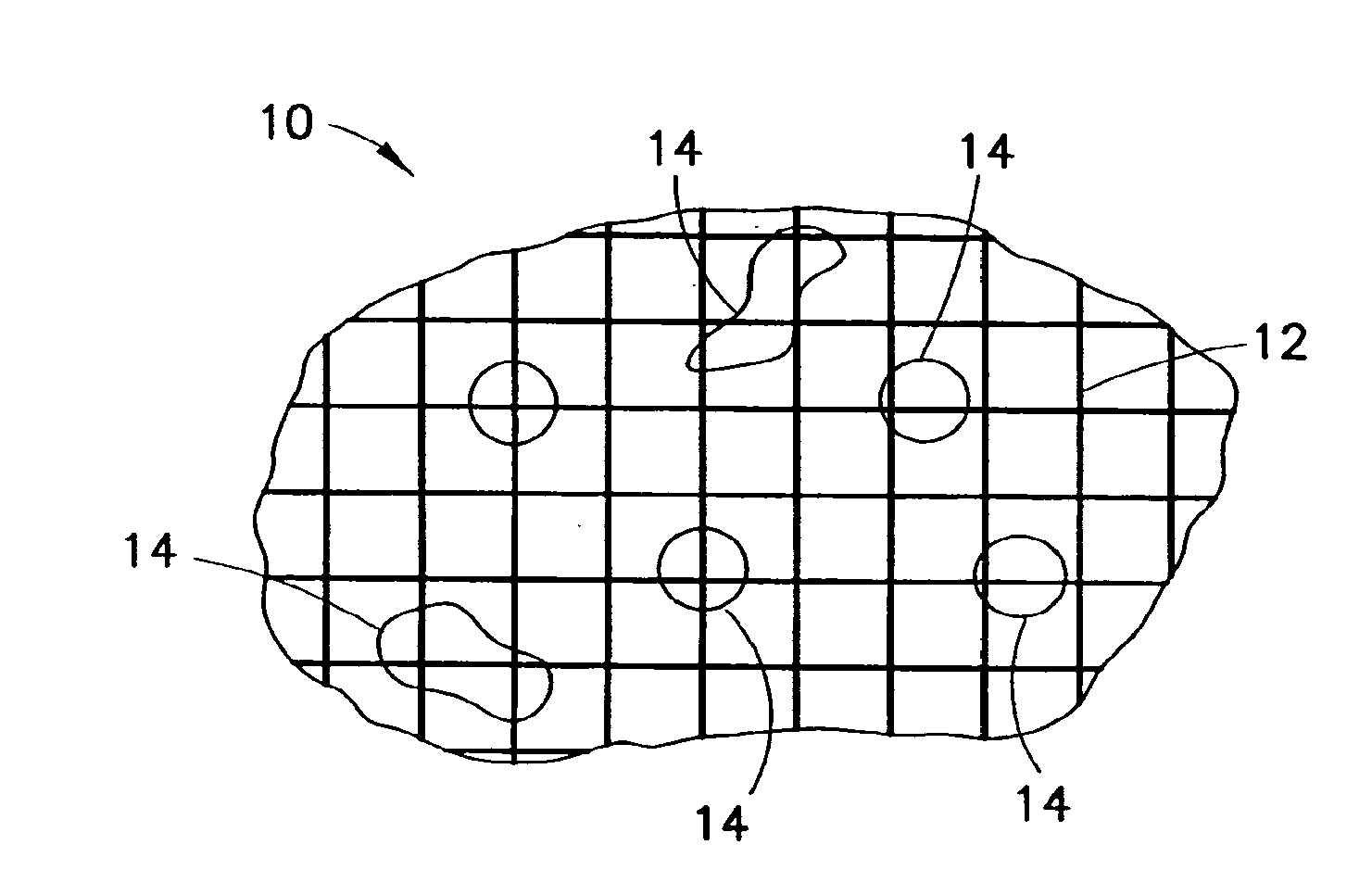
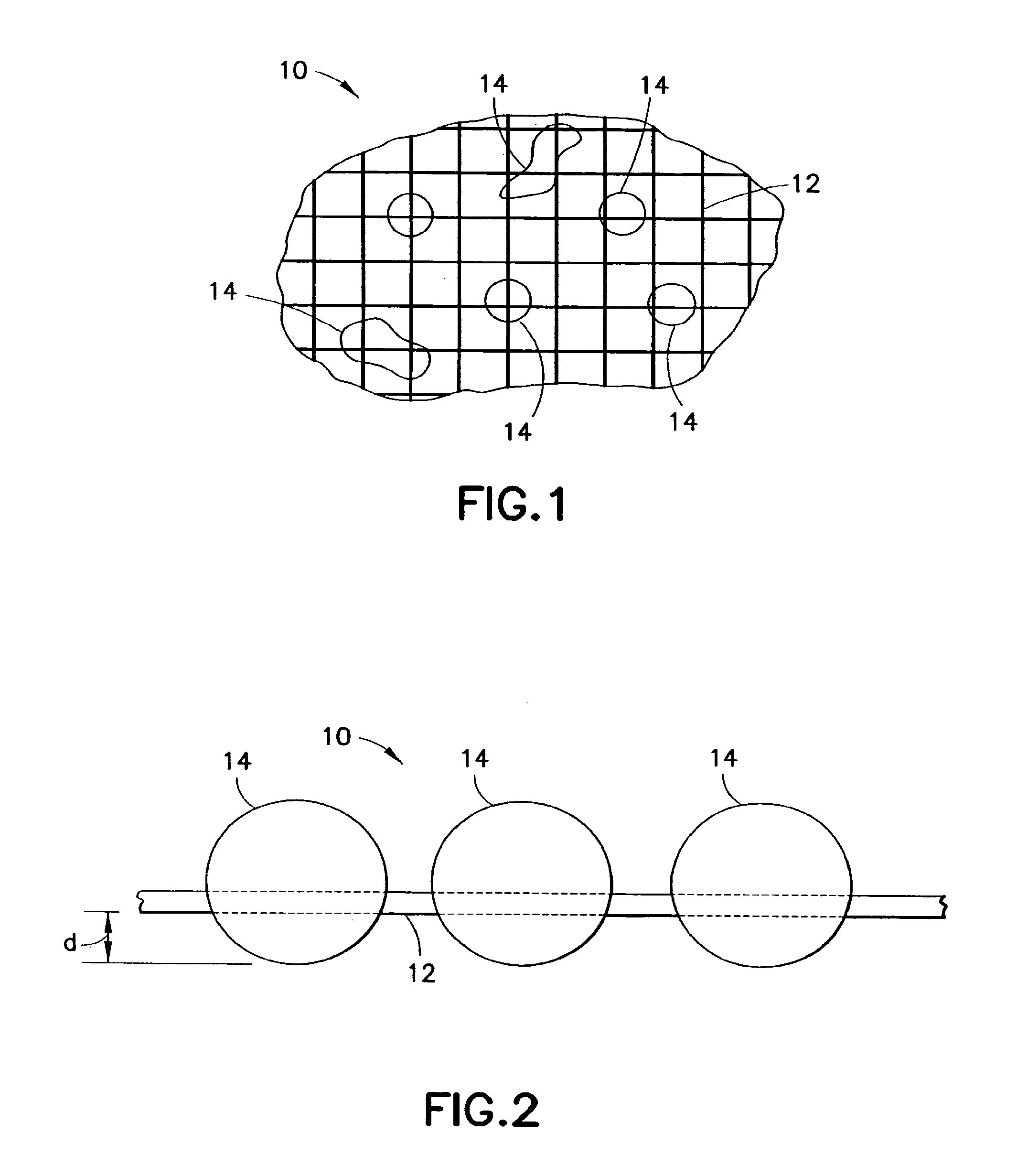
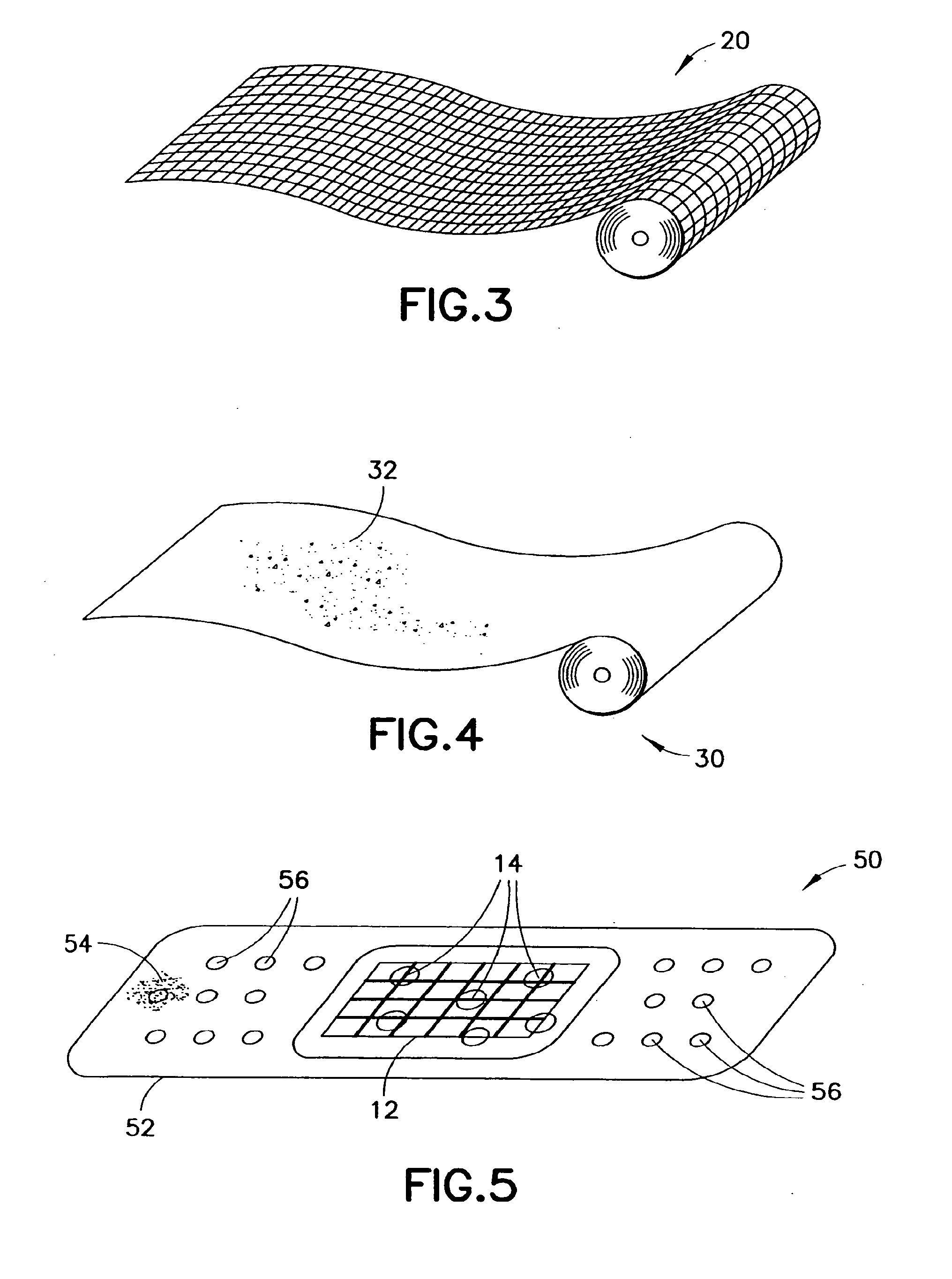
Clay-based hemostatic agents and devices for the delivery thereof
a technology of hemostasis and hemostasis agent, which is applied in the direction of antibacterial agents, drug compositions, extracellular fluid disorder, etc., can solve the problems of insufficient immediate availability of equipment and trained personnel, insufficient stanch blood flow, and insufficient hemostasis promoting effect, so as to facilitate the use of components, reduce discomfort and/or further injury to patients, and eliminate heat generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Effect of Slurry Temperature on the Ability of Cotton Gauze to Retain Kaolin Clay
[0073]Temperatures of kaolin / water slurries were varied to assess the ability of cotton gauze to retain kaolin clay. Slurries of water and EPK were prepared in which the kaolin was 40% of the total weight of the slurry. Three sponges were made (one from each piece of gauze) by immersing the cotton gauzes into the slurries of varying temperatures, rolling the wet sponges under pressure, and drying. The Table below indicates the parameters for each slurry and the results obtained.
Slurry Temp.AgitationStarting Gauzegauze weight% kaolinSample(degrees C.)methodweight (grams)after (grams)(wt. %)122Stir 13.1395.5944minute290Stir 13.0645.86848minute3100Boil 13.0856.48152minute
[0074]The gauze weight after is the weight of the gauze after rolling and drying. It was noted that the elevated slurry temperature increased the amount of retained kaolin. One theory for this is that the cotton fiber structure of the ...
example 2
Application of Dry Kaolin to Dry Cotton Gauze to Form Hemostatic Device
PUM
| Property | Measurement | Unit |
|---|---|---|
| bioactive | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com