Delivery of therapeutic and marking substance through intra lumen expansion of a delivery device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045]In the description which follows, any reference to direction or orientation is intended primarily and solely for purposes of illustration and is not intended in any way as a limitation to the scope of the present invention. Also, the particular embodiments described herein, although being preferred, are not to be considered as limiting of the present invention.
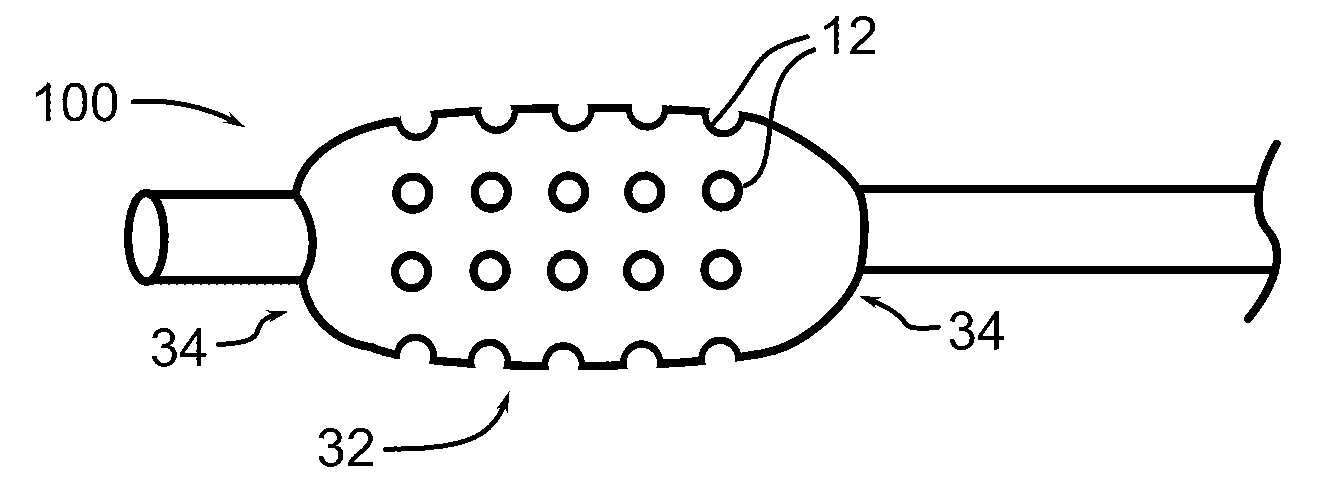
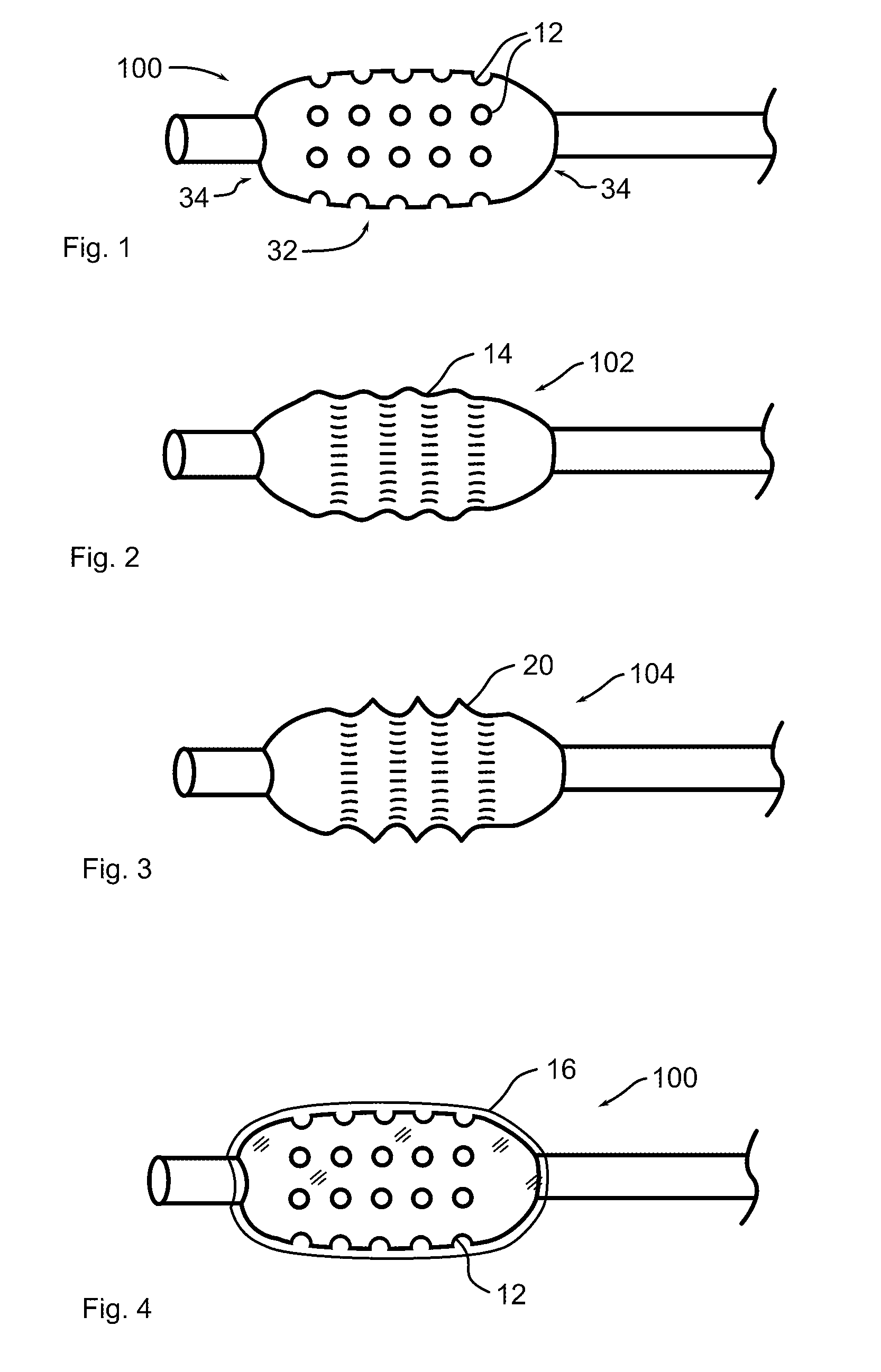
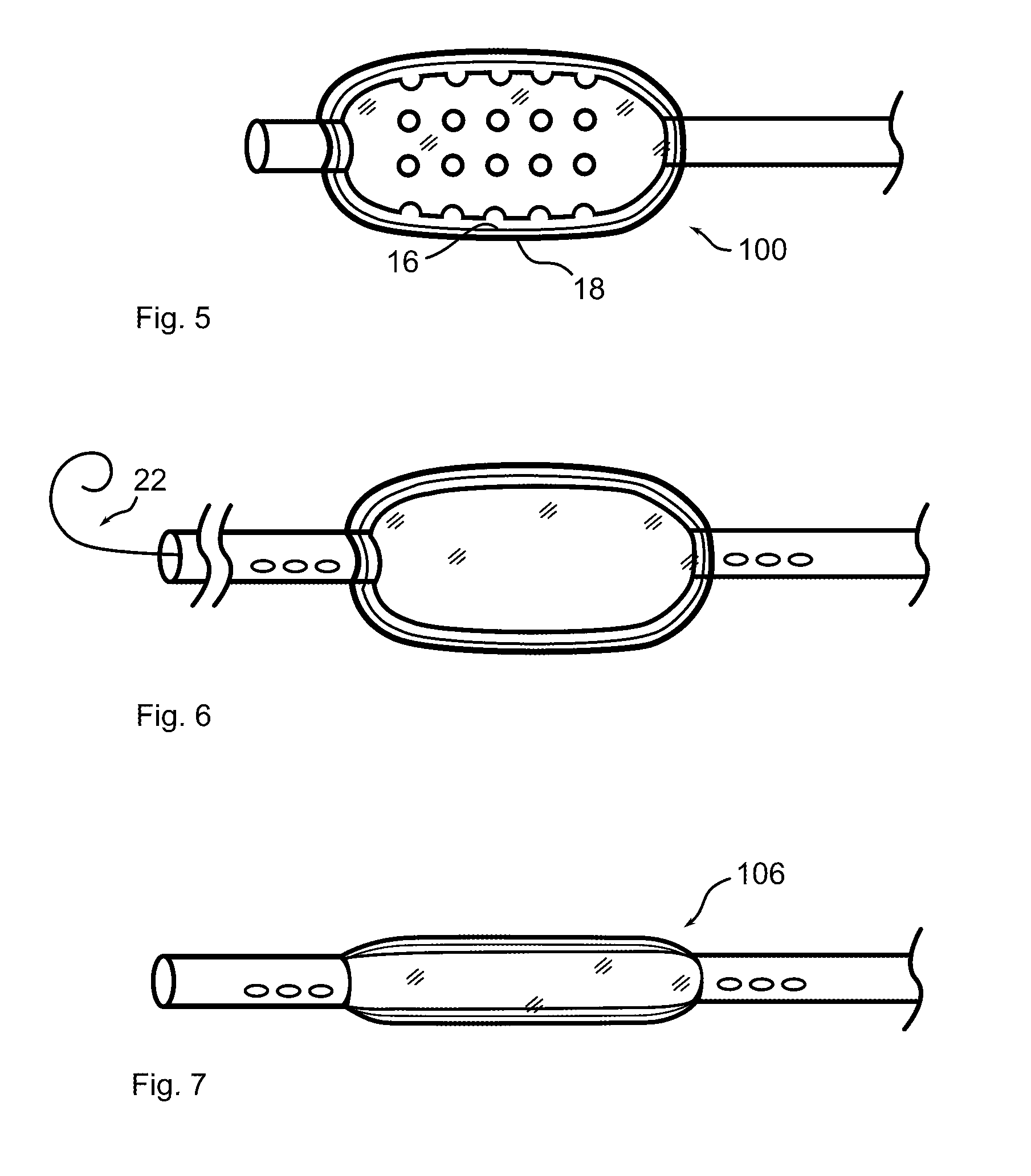
[0046]Referring now to the figures in which like reference numerals refer to like elements, an exemplary balloon catheter 100 in accordance with the invention is illustrated in FIG. 1. Balloon 100 is formed with a plurality of depressions, wells or reservoirs 12, into which a therapeutic substance is placed. When the balloon is expanded within a body lumen 200, the therapeutic substance may infuse, diffuse, elute, or physically transfer to the lumen wall. In the illustration, large reservoirs 12 are shown for clarity. In practice, reservoirs 12 may be significantly smaller and more numerous.
[0047]Balloons are typically m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com