Chimeric NK receptor and methods for treating cancer
a nk receptor and chimeric technology, applied in the field of chimeric nk receptor and methods for treating cancer, can solve the problems of time-consuming, laborious and sometimes difficult, and the isolation and expansion of t cells that retain their antigen specificity and function can also be a challenging task, so as to reduce or eliminate tumors, reduce the regulatory t cell population in the subject, and reduce the regulatory t cell population
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Mice and Cell Lines
[0069]C57BL / 6 mice were purchased from the National Cancer Institute, and all animal work was conducted in accordance with standard guidelines.
[0070]Cell lines Bosc23, PT67, GP+E86, EG7 (H-2b), and YAC-1 were obtained from the American Type Culture Collection (ATCC, Rockville, Md.). RMA cells (H-2b) originated from a Rauscher virus-induced C57BL / 6 T-cell lymphoma (Ljunggren and Karre (1985) J. Exp. Med. 162:1745-1759). RMAS-S is a sub-line of RMA which lacks MHC class-I surface expression (Karre, et al. (1986) Nature 319:675-678). All packaging cells were grown in Dulbecco's modified Eagle medium (DMEM) with a high glucose concentration (4.5 gram / liter) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, Utah), 20 U / mL penicillin, 20 μg / mL streptomycin, 1 mM pyruvate, 10 mM HEPES, 0.1 mM non-essential amino acids, 50 μM 2-mercaptoethanol. RMA, EG7, RMA-S and YAC-1 cells were cultured in RPMI plus the same supplements described above.
example 2
Retroviral Vector Construction
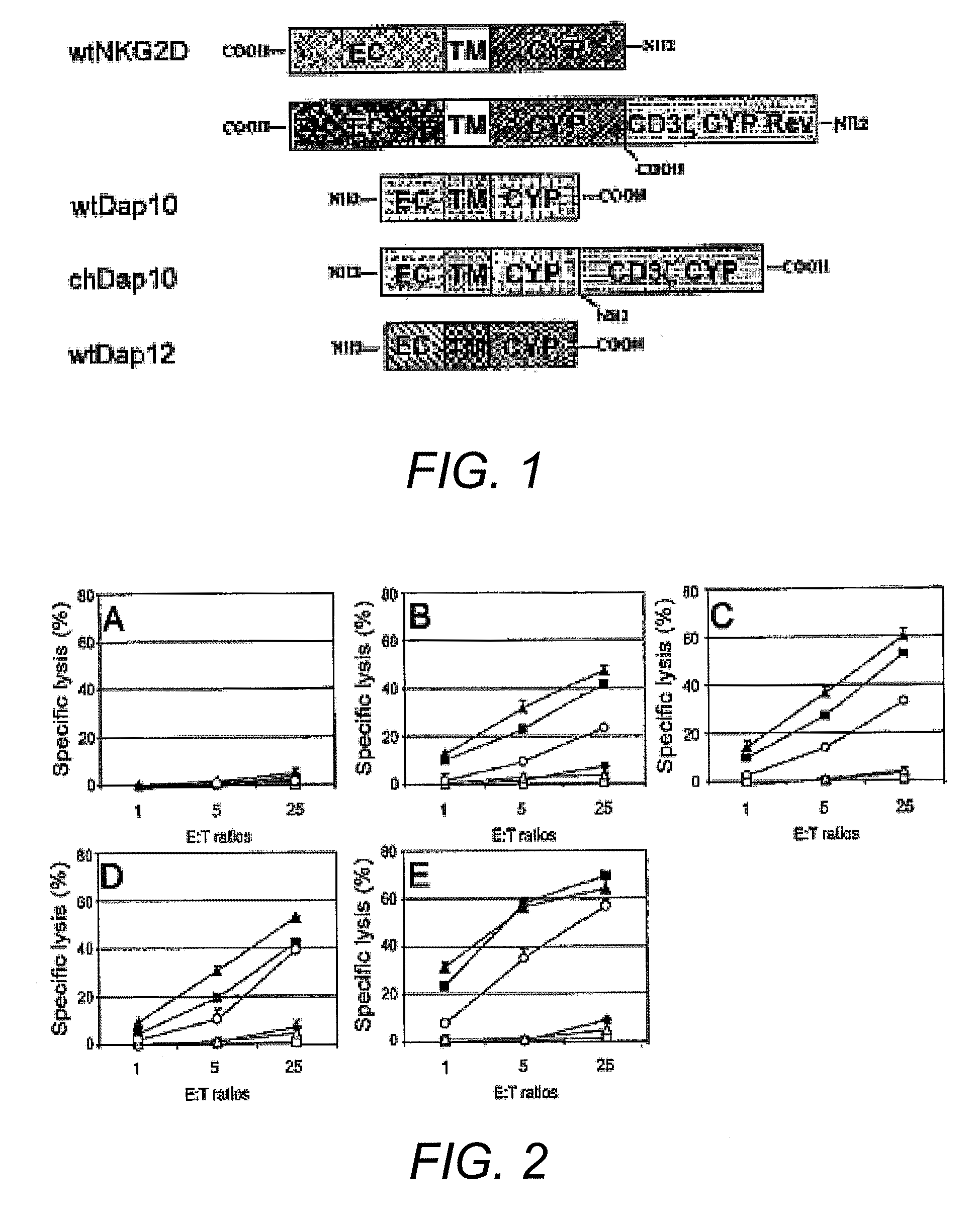
[0071]The full-length murine NKG2D cDNA was purchased from Open Biosystems (Huntsville, Ala.). Murine CD3ζ chain, Dap10 and Dap12 cDNAs were cloned by RT-PCR using RNAs from ConA- or IL-2 (1000 U / mL)-activated spleen cells as templates. Mouse NKG2D ligands Rae-11 and H60 were cloned from YAC-1 cells by RT-PCR. All PCR reactions were performed using high-fidelity enzyme Pfu or PFUULTRA™ (STRATAGENE®, La Jolla, Calif.). The oligonucleotides employed in these PCR reactions are listed in Table 9.
TABLE 9SEQIDNo.PrimerSequenceNO:15′ wtNKG2DGCGAATTCGCCACCATGGCATTGATTCGTGATCGA823′ wtNKG2DGGCGCTCGAGTTACACCGCCCTTTTCATGCAGAT935′ chNKG2DGGCGAATTCGCATTGATTCGTGATCGAAAGTCT1045′ wtDAP10GCAAGTCGACGCCACCATGGACCCCCCAGGCTACC1153′ wtDAP10GGCGAATTCTCAGCCTCTGCCAGGCATGTTGAT1263′ chDAP10GGCAGAATTCGCCTCTGCCAGGCATGTTGATGTA1375′ wtDAP12GTTAGAATTCGCCACCATGGGGGCTCTGGAGCCCT1483′ wtDAP12GCAACTCGAGTCATCTGTAATATTGCCTCTGTG1595′ ATG-CD3ζGGCGTCGACACCATGAGAGCAAAATTCAGCAGGAG16103′ ATG-CD3ζGC...
example 3
Retrovirus Production and Transduction
[0074]Eighteen hours before transfection, Bosc23 cells were plated in 25 cm2 flasks at a density of 4×106 cells per flask in 6 mL of DMEM-10. Transfection of retroviral constructs into Bosc23 cells was performed using LIPOFECTAMINE™ 2000 (INVITROGENT™, Carlsbad, Calif.) according to the manufacturer's instruction. Viral supernatants were collected 48 and 72 hours post-transfection and filtered (0.45 μm) before use. For generation of large scale, high-titer ecotropic vectors, the ecotropic viruses produced above were used to transduce the dualtropic packaging cell PT67 in the presence of polybrene (8 μg / mL). After three rounds of transduction, PT67 cells were selected in G418 (1 mg / mL) for 7 days. Dualtropic vectors were then used to transduce ecotropic cell line GP+E86. Through this process, the virus titer from pooled GP+E86 cells generally was over 1×106 CFU / mL. Concentration of retroviruses by polyethylene glycol (PEG) was performed according...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com