Novel antibiotic alternatives
a technology of antibiotics and alternatives, applied in the field of new antibiotic alternatives, can solve the problems of not being cost-effective to purify this protein, and death of cellular targeted bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Colicin Production, Purification, and Efficacy
Colicin Production and Purification
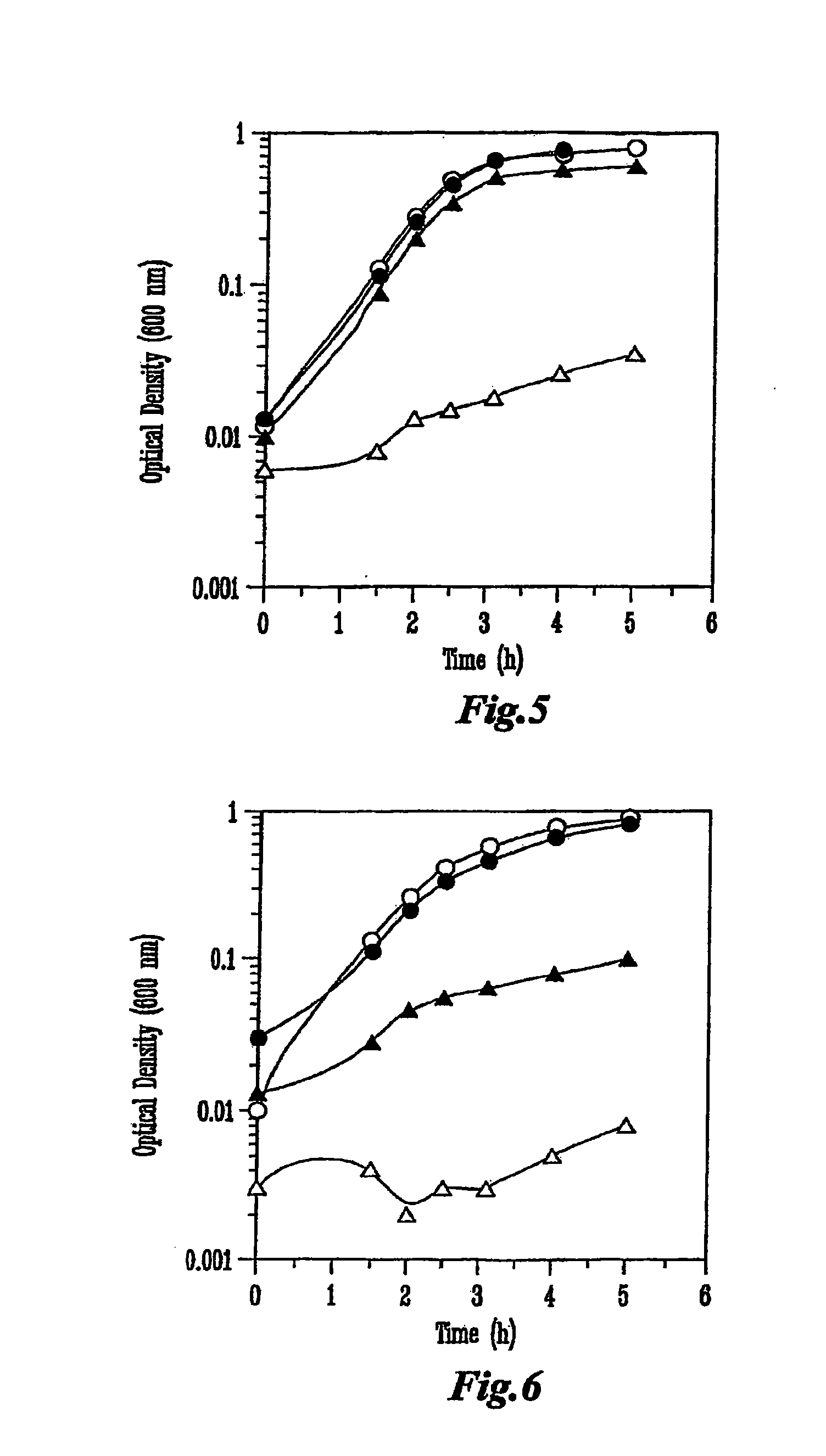
[0069]Colicin producing E. coli strains, obtained from the National Collection of Type Cultures (Public Health Laboratory Service, London, England), were inoculated into Luria Broth (LB) to a starting OD600˜0.1, and incubated with shaking at 37° C. When the cultures reached an OD600=0.9 colicin production was induced by the addition of 0.2 U Mitomycin C (Sigma) / mL culture. The cell free supernatant was obtained by centrifugation 5.5 h later, and concentrated by ultrafiltration in a stir-cell apparatus (Amicon, Millipore, Bedford, Mass.) across a regenerated cellulose membrane with a 30 kDa cut-off (Millipore). The concentrated sample was then desalted against 10 mM Tris-Cl, pH 8 and purified by ion exchange chromatography. The desalted samples were applied to a column containing Q Sepharose (Amersham Biosciences, Piscataway N.J.), equilibrated with 10 mM Tris-Cl, pH 8.0 (equilibration buffer), and exhau...
example 2
Inhibition of Growth of Escherichia coli 0157:H7 In Vitro
Materials and Methods
Colicin Production and Purification
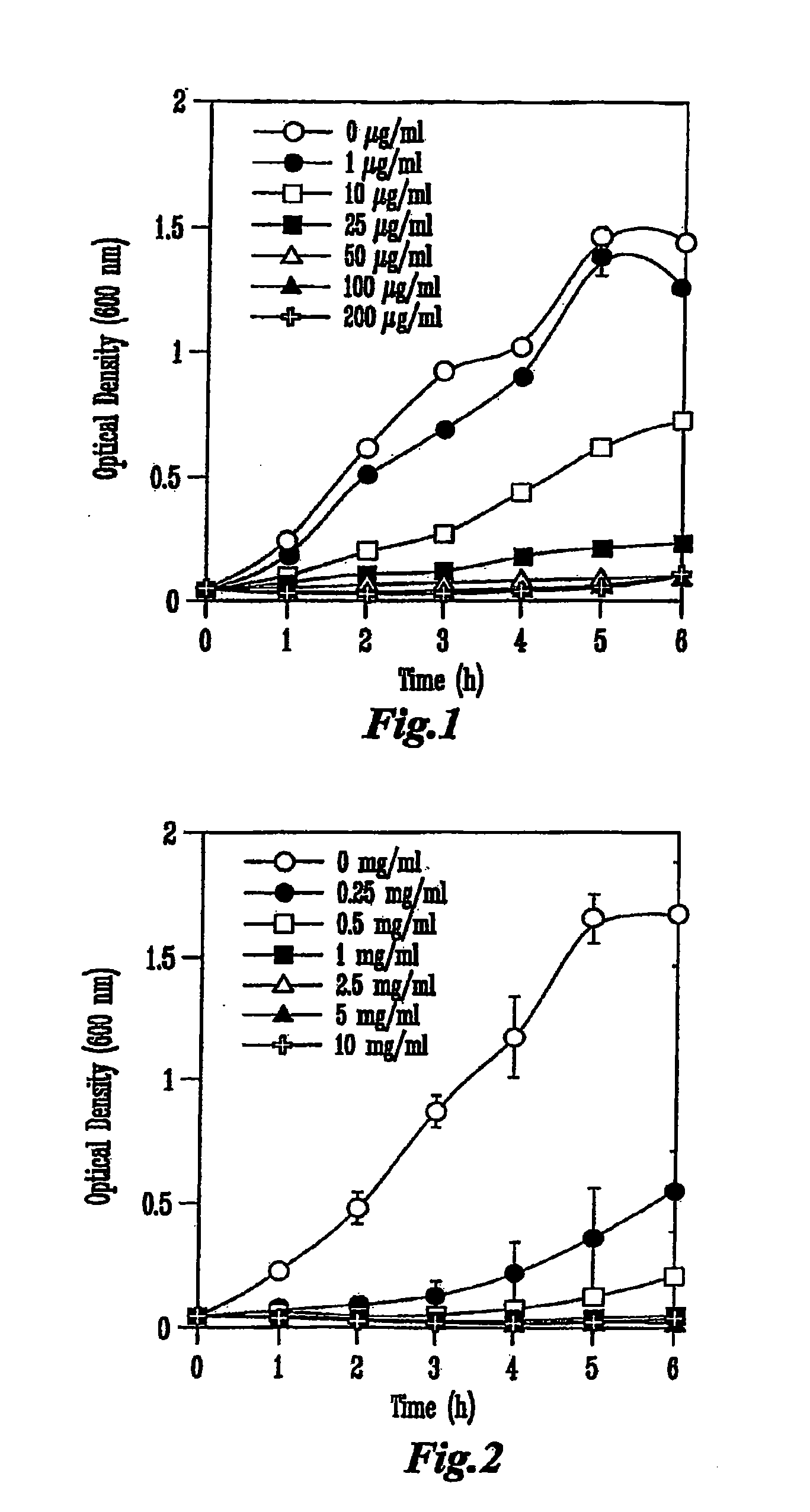
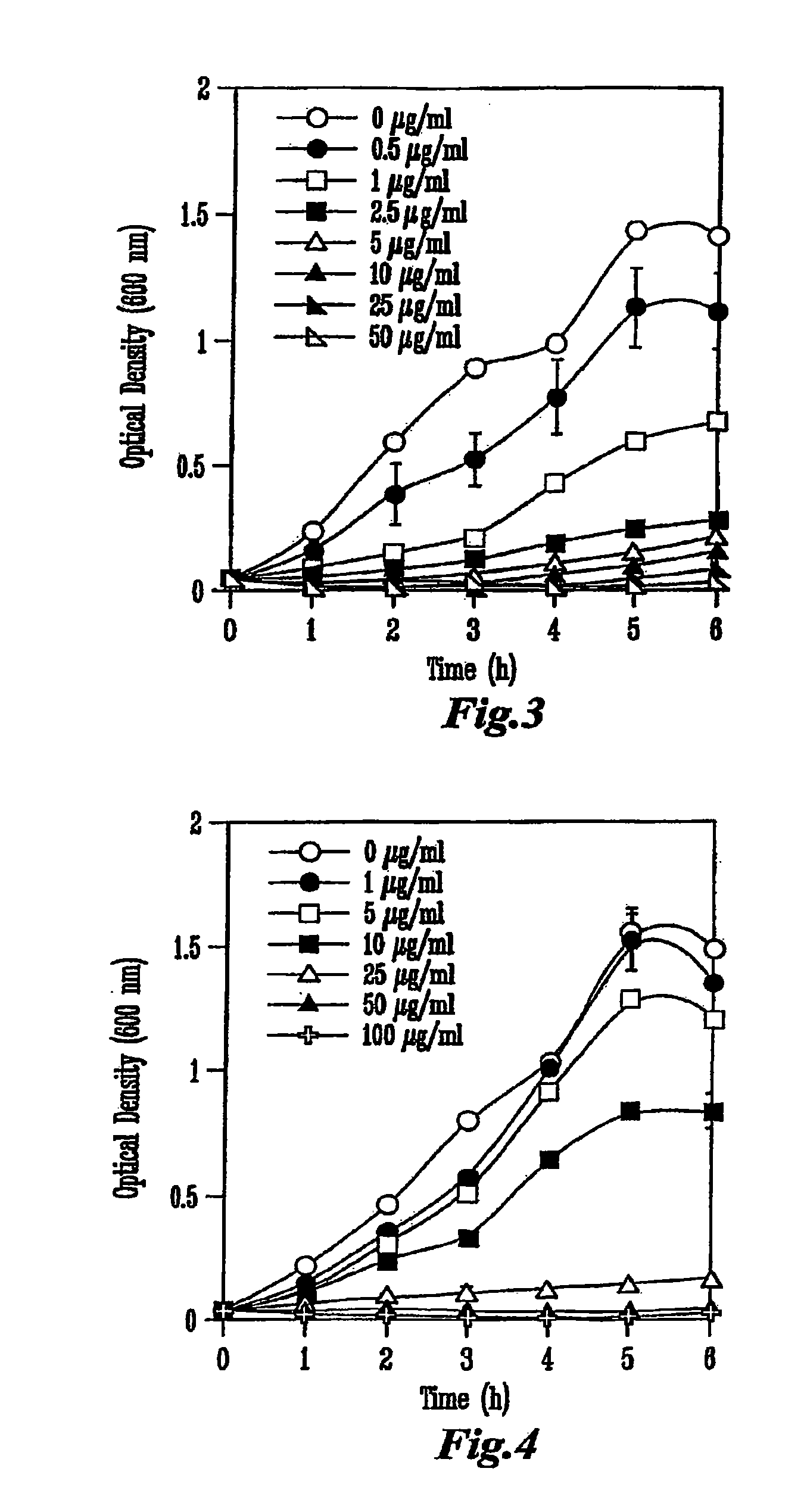
[0076]Each colicin was produced from a specific colicin-producing E. coli K-12 strain (NC50129-01 containing plasmid pColA-CA31, NC50132-01 containing plasmid pColE1-K53, and NC50145-01 containing plasmid pColN-284) obtained from the National Collection of Type Cultures (Public Health Laboratory Service, London, UK). Cultures were inoculated into Luria-Bertani (LB) broth to an initial optical density of 600 nm (OD600) of approximately 0.1 and incubated in a shaker at 37° C. When the cultures reached OD600=0.9, colicin production was induced by the addition of 0.2 U of mitomycin C per ml of culture (Sigma Chemicals, St. Louis, Mo.). The cell-free supernatant was obtained by centrifugation 5.5 h later and concentrated by ultrafiltration in a stir-cell apparatus (Amicon, Millipore, Bedford, Mass.) across a regenerated cellulose membrane with a 30-kDa cut-off (Millipore). The...
example 3
Recombinant Expression of Colicins
[0088]Yeast expression vectors were constructed and verified by sequencing for Colicin A, B, E1, 1a, and N. Expression and secretion of an active Colicin A by Pichia pastoris were obtained, and confirmed by spot testing of cell-free supernatant of Pichia pastoris expressing Colicin A against E. coli DH5α. In the functional assay (spot testing) 10 μl of cell-free supernatant from transformed P. pastoris showed a clearing of approximately the same size as 1 μg of purified Colicin A from E. coli. Spot 2 showed 1 μl of cell-free supernatant from P. pastoris. Spot 3 showed 5 μl of cell-free supernatant from P. pastoris, and spot 4 showed 10 μl of cell-free supernatant from P. pastoris.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com