Lyophilized DNA Formulations for Enhanced Expression of Plasmid DNA
a technology of plasmid dna and lyophilized dna, which is applied in the direction of drug compositions, genetic material ingredients, cardiovascular disorders, etc., can solve the problems of statistically significant loss of transfection efficiency, reduced gene expression efficiency, and formulations that are not lyophilized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Plasmid
[0078]The plasmid pCK-HGF-X7 (WO 03 / 078568) which is designed to express hepatocyte growth factor (HGF) protein was used in the experiment.
[0079]E. coli (TOP10, Invitrogen, USA) were transformed with pCK-HGF-X7, and a single colony was isolated. The isolated colony was then cultured in LB media containing 30 μg / mL kanamycin. Plasmid DNA was purified using an EndoFree plasmid Giga kit (Qiagen, USA), and re-suspended in saline containing 0.9% NaCl at a final DNA concentration of 1.0 to 2.0 mg / mL.
example 2
Lyophilization
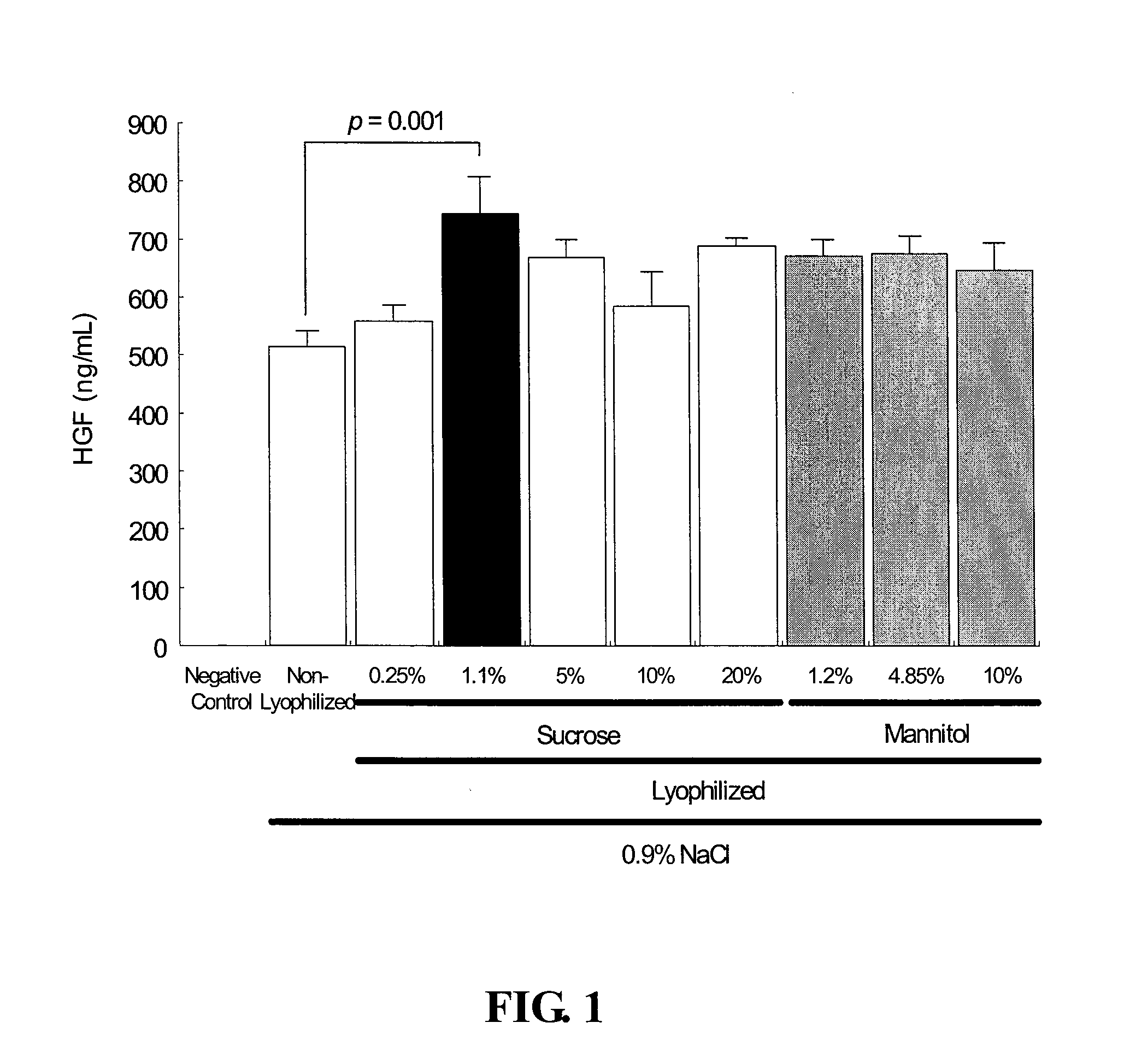
[0080]Formulations of pCK-HGF-X7 were prepared in saline containing 0.9% NaCl at a final DNA concentration of 0.5 mg / mL or 1 mg / mL, with sucrose (0.25, 1.1, 5, 10 or 20% w / v) or mannitol (1.2, 4.85 or 10% w / v). Table 1A and 1B show the percentage sucrose and mannitol, respectively, and the corresponding carbohydrate / DNA (w / w) ratios for the tested pCK-HGF-X7 formulations.
TABLE 1APercent SucroseDNASucroseSucroseSucrose(mg / ml)(%)(mg / ml))to DNA ratio (w / w)0.50.252.550.51.111220.55501000.5101002000.52020040010.252.52.511.11111155050110100100120200200
TABLE 1BPercent MannitolDNAMannitolMannitolMannitol(mg / ml)(%)(mg / ml))to DNA ratio (w / w)0.51.212240.54.8548.5970.51010020011.2121214.8548.548.5110100100
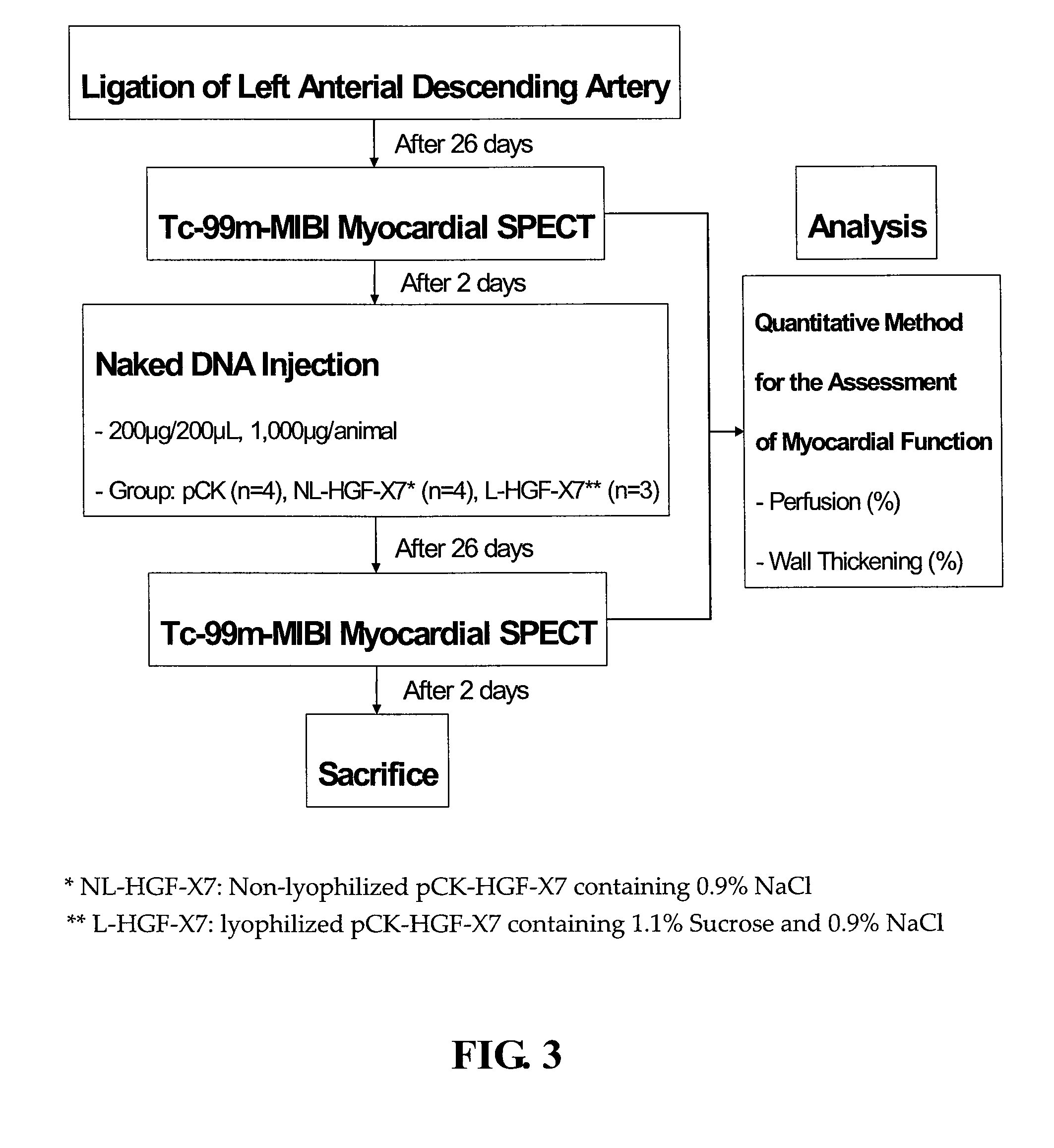
[0081]The suspended plasmid DNA was then lyophilized with Production-Master Freeze Dryer (C&H Cooling & Heating Systems, Korea). The temperature was lowered to −50° C. for 4 hours at 100 mTorr. Then, the temperature was raised to −40° C. for 12 hours, −30° C. for 6 hours, −20° C....
example 3
Effects of Lyophilization on In Vitro Gene Expression Efficiency of Plasmid DNA
[0085]1. Materials and methods
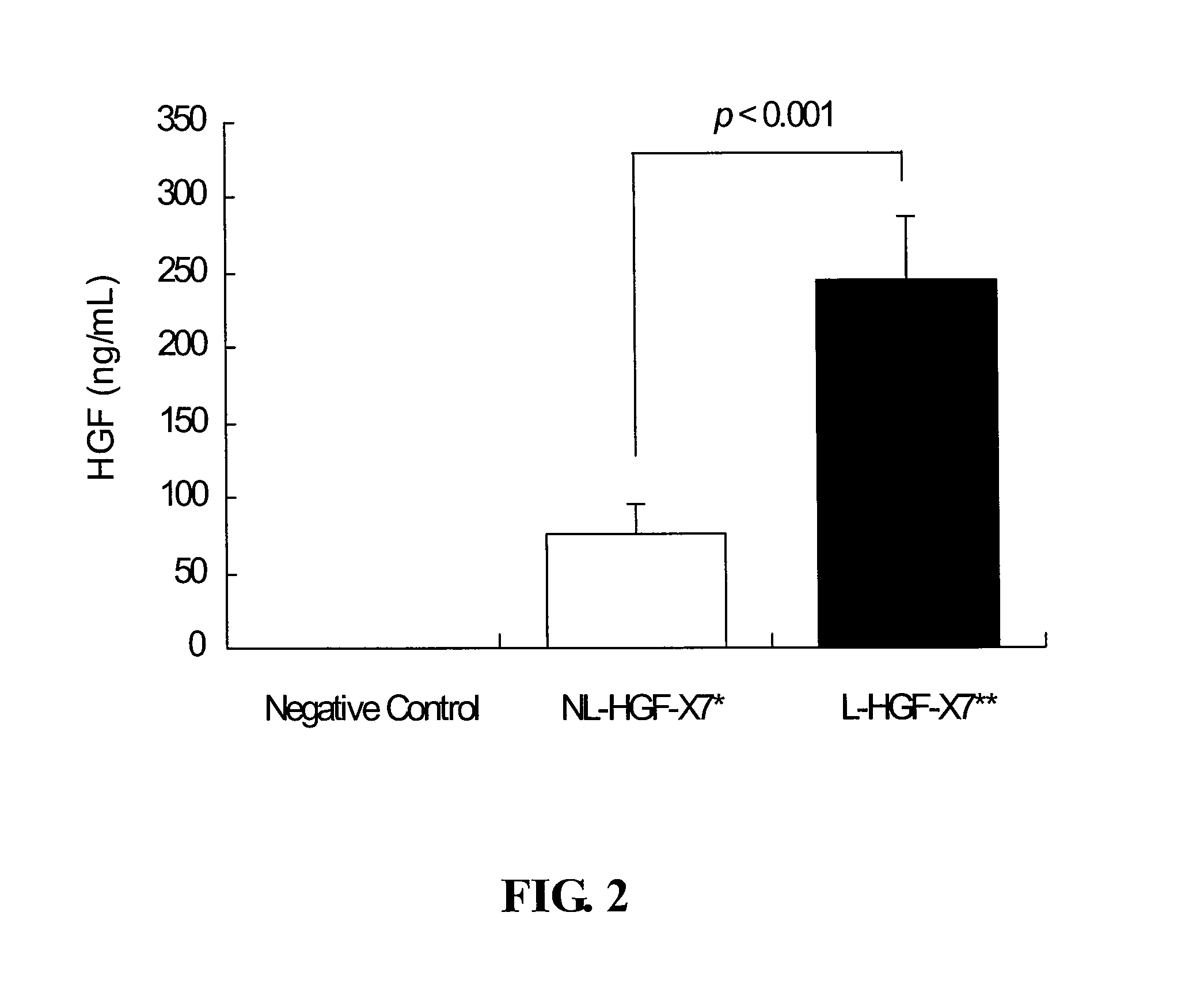
[0086]To assess the effects of the lyophilization on gene expression efficiency of plasmid DNA, the lyophilized plasmid DNA was transfected into 293T cells, and the level of HGF expression was measured. As a control, non-lyophilized plasmid DNA was also transfected.
[0087]Four micrograms of pCK-HGF-X7 in various formulations (as noted above in Example 1) were transfected into 1×106 293T cells using FuGENE6 (Roche Diagnostics, Germany) (n=5). Before transfection, 1 mg of the lyophilized plasmid DNA was reconstituted with 2 ml of water for injection to the final concentration of 0.5 mg / mL.
[0088]Two days after transfection, the culture supernatants were obtained and analyzed for HGF expression using a human HGF ELISA kit (R&D Systems, MN, USA), according to the manufacturer's recommendations. The ELISA results were statistically assessed by Dunnett's multiple comparison test usin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com