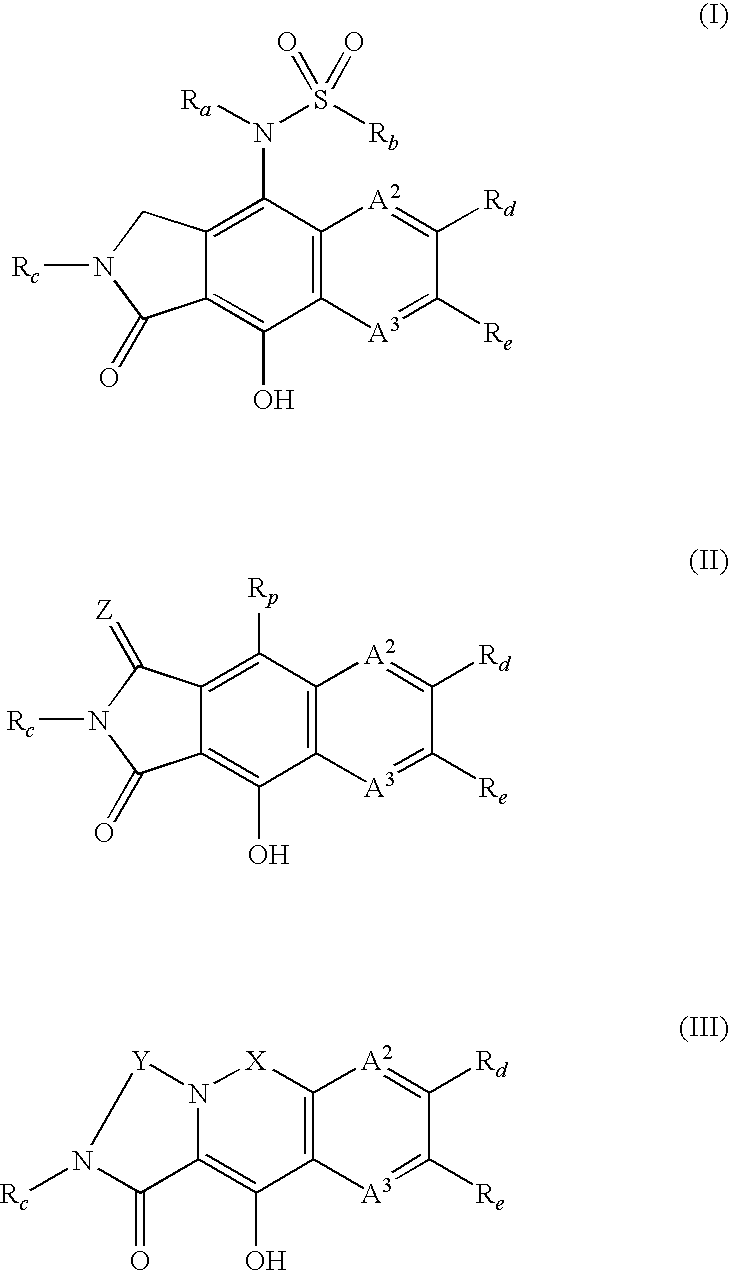
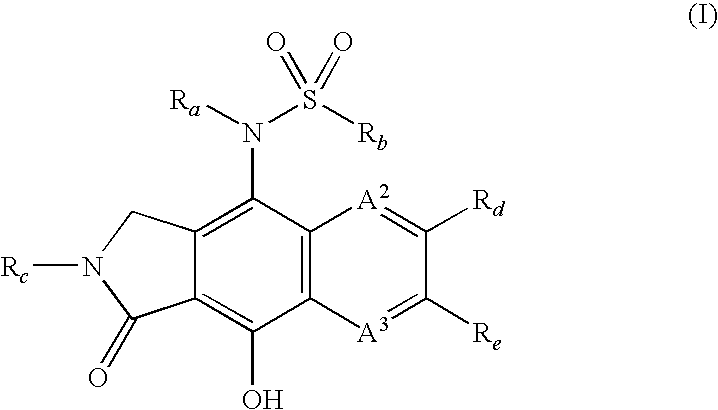
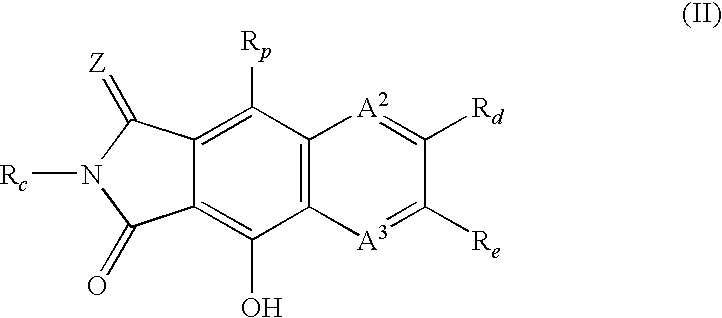
Integrase inhibitors
a technology of integrase inhibitors and inhibitors, which is applied in the field of compounds having antiviral activity, can solve the problems of inability to inhibit the activity of integrase inhibitors, the inability to fully integrate preintegration complexes, and the inability to inhibit the inhibitory potency of fully assembled preintegration complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0331]
[0332]Compound (1) (107 mg, 213 μmol) was dissolved in 5 mL of pyridine and flashed with nitrogen, It was cold to 0° C. and added sulfonyl chloride (200 μl) and stirred for 2 h under nitrogen. It became dark. The reaction was diluted with 20 mL of EtOAc, washed with 0.1N HCl, brine, sat'd NaHCO3 and brine again. It was dried over Na2SO4 and filtered through a pile of Celite, then concentrated in vacuum to give crude product (2). The crude product was purified by flash chromatography with 30% EtOAc / Hexane to yield 81 mg of desired product in 62%.
General Procedure for the Deprotection of DPM Group at C8-OH:
[0333]The compound (2) (20 mg) was dissolved in 1 mL of DCM and treated with TFA (100 μl) and triethylsilane (200 μl). After stirring for 30 minutes at room temperature, the reaction mixture was azeotroped with toluene once. The resulting residue was then purified by reverse-phase prep HPLC to provide 11.5 mg of (3), as the TFA salt.
[0334]General HPLC conditions: mobile phase ...
example 2
[0336]
[0337]9 (100 mg, 0.18 mmol) was dissolved in 1.0 ml of THF. Cyclopropylmethanol (21.3 ul, 0.27 mmol), Triphenylphosphine (71 mg, 0.27 mmol), and DIAD (53 uL, 0.27 mmol) were then added to this solution successively and the reaction was allowed to stir at room temperature for 45 minutes. The reaction was then diluted with Ethyl Acetate and the organic was washed once with sat. NaHCO3, twice with water, and once with Brine. The organic was then dried over Magnesium Sulfate and concentrated in vacuo to afford a crude residue which was then purified by silica gel chromatography (3:1—Hexane:EthylAcetate) to afford 10 (90 mg, 81%).
example 3
[0338]
[0339]9 (100 mg, 0.18 mmol) was dissolved in 1.0 ml of THF. N,N-Dimethylaminoethanol (81 ul, 0.8 mmol), Triphenylphosphine (210 mg, 0.8 mmol), and DIAD (155 ul, 0.8 mmol) were then added to this solution successively and the reaction was allowed to stir at room temperature for 45 minutes. The reaction was then diluted with Ethyl Acetate and the organic was washed once with sat. NaHCO3, twice with water, and once with Brine. The organic was then dried over Magnesium Sulfate and concentrated in vacuo to afford a crude residue which was then purified by silica gel chromatography (1% Triethylamine in EthylAcetate) to afford 13 (84 mg, 72%).
[0340]13 (84 mg, 0.13 mmol) was dissolved in 1 ml of THF and 100 ul of water. LiBH4 (22 mg, 1.05 mmol) was then added and the reaction then stirred at room temperature for 1 hour. The reaction was then diluted with Ethyl Acetate and the organic was washed once with water and once with Brine. The organic was then dried over Mg2SO4 and concentrate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| particle sizes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com